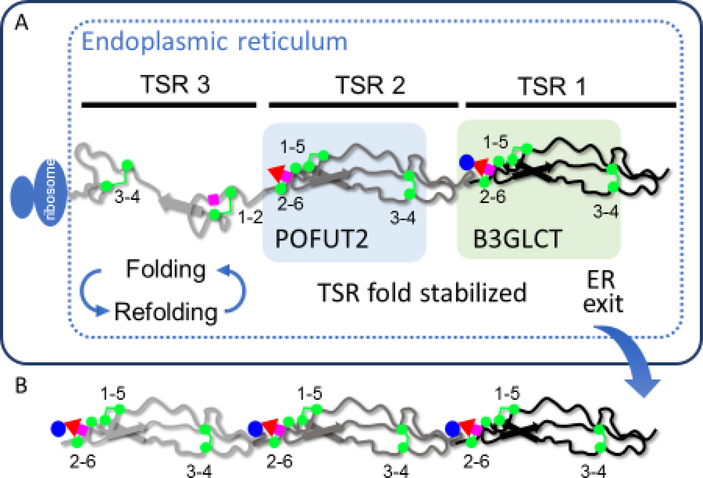
Figure 3. O-Fucosylation stabilizes TSRs by interacting with underlying amino acids.
A. A hypothetical protein with 3 TSRs is being translated and is folding in the ER. TSR3 is in the folding cycle, with some correct and some incorrect disulfide bonds. TSR3 is not modified by O-fucose glycans. TSR2 is fully folded, stabilized by addition of fucose by POFUT2, and is no longer part of a folding cycle. TSR1 is further stabilized by addition of glucose by B3GLCT. Note that the folding pathway for a TSR is not known, so this pathway is hypothetical. B. Once all TSRs with a POFUT2 consensus sequence are modified with Glucoseβ1–3Fucose disaccharide and the protein is fully folded, the native protein exits the ER, transits the secretory system, and is either directed to the cell surface or secreted from the cell depending upon the target protein properties.