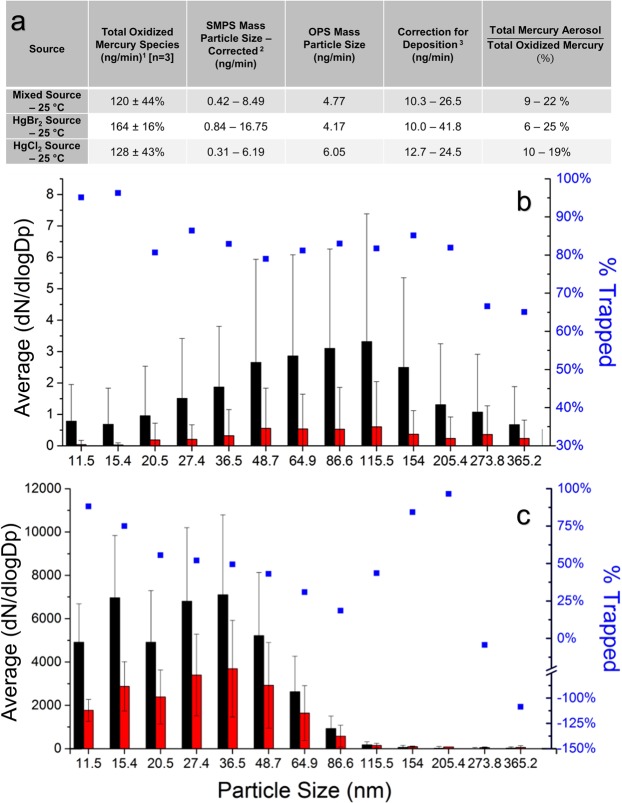
Figure 2.
(a) Total oxidized mercury from halogenated mercury sources. (1) The KCl Denuder – CVAFS measured the mass of Hg which for HgBr2 and HgCl2 were corrected to include the mass of the Br and Cl atoms, respectively. The correction for the mixed source was based on the relative ratio between the vapour pressures of mercuric bromide and mercuric chloride at 25 °C. (2) The SMPS unipolar charger typically ionizes between 1% and 20% of particles. As sizing is based on electron mobilities, a charge is required for counting. Ranges for corrected values are given based on this ionization efficiency. (3) At the highest concentrations and highest temperatures, deposition on tubing downstream from the source was observed. A conservative estimate for deposition (1:1) was chosen based on comparable particle counts for deposits and aerosols in Nanoparticle Tracking analysis. (b) Denuder capture of synthetic nano-sized mercuric bromide particles produced by vapour condensation method. Particle size distributions for nano-sized mercuric bromide particles produced by vapour flow condensation (shown in black) and those produced by vapour flow condensation and subsequently passed through a KCl denuder (shown in red). Flow streams passed through conductive tubing of equal length before entering the SMPS inlet at a flow rate of 3 LPM. (n = 70). (c) Denuder capture of synthetic nano-sized mercuric chloride particles produced from aqueous nebulization. Particle size distributions for nano-sized mercuric chloride particles produced by aqueous solution nebulization (shown in black) and those produced by vapour flow condensation and subsequently passed through a KCl denuder (shown in red). Flow streams passed through conductive tubing of an equivalent length before entering the SMPS inlet at a flow rate of 3 LPM. (n = 70).