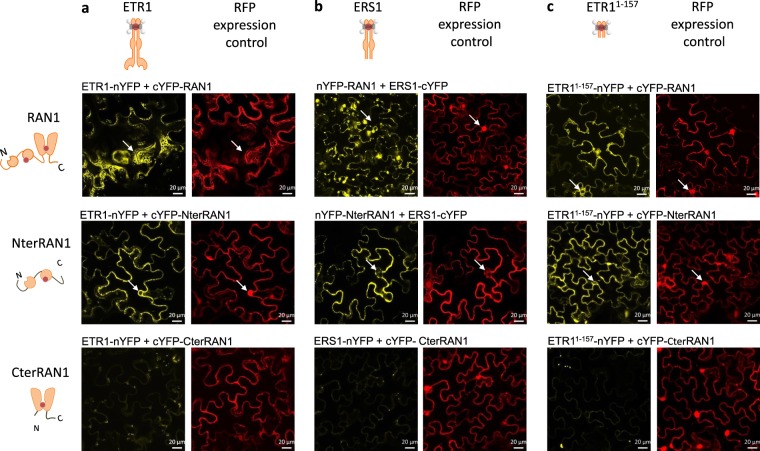
Figure 3.
In planta Bimolecular Fluorescence Complementation (BiFC) studies on the interaction of RAN1 with type-I ethylene receptors ETR1 and ERS1. BiFC studies on RAN1 and deletion mutants reveal an in vivo interaction of RAN1 with receptors ETR1 (A) and ERS1 (B). Complementation is observed for full-length RAN1 and NterRAN1 but not for CterRAN1 indicating that the large N-terminal region of RAN1 mediates the interaction. Additionally, deletion mutant ETR11–157 (C) was tested which also interacts with RAN1 and NterRAN1 demonstrating that the transmembrane part of the receptor seems to be sufficient for interaction. RFP expression acts as an infiltration control for the BiFC vector and is constitutively expressed (d35S). Constructs containing full-length ETR1 were under control of an inducible promoter whilst constructs containing ERS1 and ETR11–157 were constitutively expressed (d35S). Arrows indicate the meshed like structure or nucleus envelope staining, typical for the ER, visible only for the complementation signal (YFP) but not for free RFP. nYFP and cYFP symbolize the YFP fragment fused either N- or C-terminal to the protein of interest.