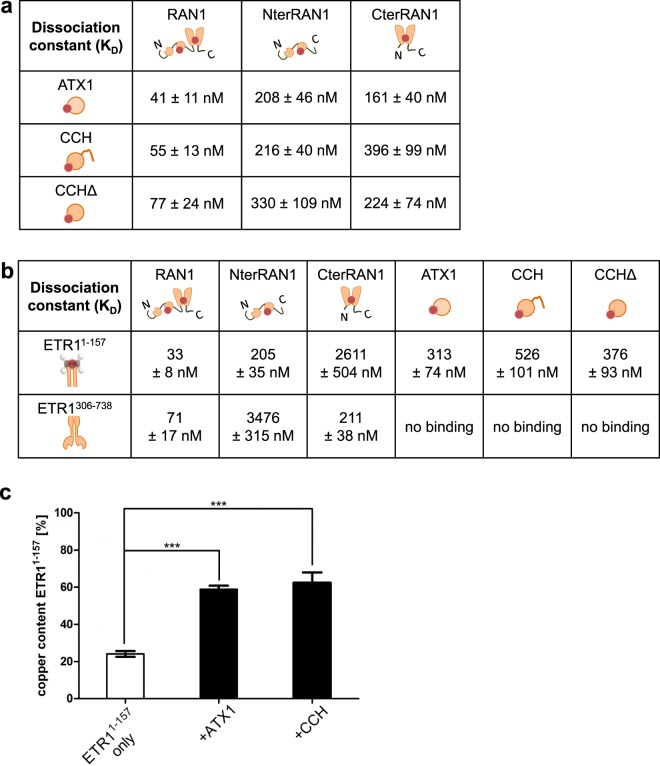
Figure 4.
In vitro binding studies on RAN1, ETR1 and soluble copper chaperones by microscale thermophoresis (MST) (A) Dissociation constants of RAN1 and RAN1 truncation mutants with soluble copper chaperones ATX1, CCH and CCHΔ, respectively. (B) Dissociation constants of ETR11–157 and ETR1306–738 with copper carriers RAN1, NterRAN1, CterRAN1, ATX1, CCH or CCHΔ, respectively. ETR1 interacts with both, the ATX-like chaperones and RAN1. Noteworthy, affinity of ETR1 for soluble copper chaperones is 10 fold lower than observed for ETR1 with full-length RAN1. For detailed binding curves see Fig. S3. (C) Copper transfer assay with purified recombinant Cu(I)-ATX1 or Cu(I)-CCH and ETR11–157. Copper content of ETR11–157 before (ETR11–157 only) and after (+ATX1, +CCH) incubation with preloaded donor copper chaperone. Increase in copper loading after chaperone incubation indicates chaperone mediated copper transfer to ETR1. Asterisks indicate significance level (p ≤ 0.001) determined using Student’s t-test.