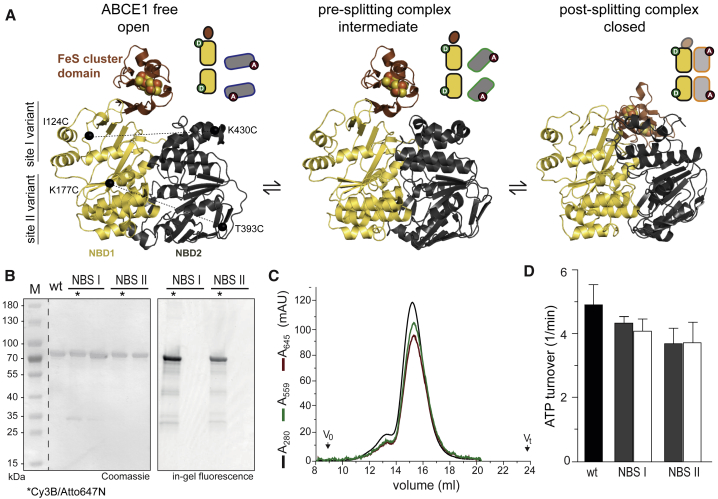
Figure 1.
Biochemical Function of FRET Pair-Labeled ABCE1
(A) Double-cysteine variants probing the conformational states of site I (ABCE1I124C/K430C) or site II (ABCE1K177C/T393C) via smFRET are depicted on the crystal structures of ABCE1 (Barthelme et al., 2011, Karcher et al., 2008). Middle: cryo-EM structure of the ribosome-bound (pre-splitting complex) intermediate state of ABCE1 (Becker et al., 2012). Right: cryo-EM structure of the closed (ATP bound) state bound to the small ribosomal subunit (post-splitting complex) (Heuer et al., 2017).
(B) ABCE1 wild type and variants were purified to homogeneity by metal affinity and anion exchange chromatography. SDS-PAGE (12.5%, Coomassie and in-gel fluorescence). Donor and acceptor fluorophores are illustrated as D and A, respectively.
(C) FRET pair-labeled ABCE1 variants were analyzed by fluorescence-based size-exclusion chromatography (SEC) and subsequently used for smFRET.
(D) ATPase activity of ABCE1 before (gray) and after (white) fluorescence labeling. Data represent mean ± SD from three independent experiments.