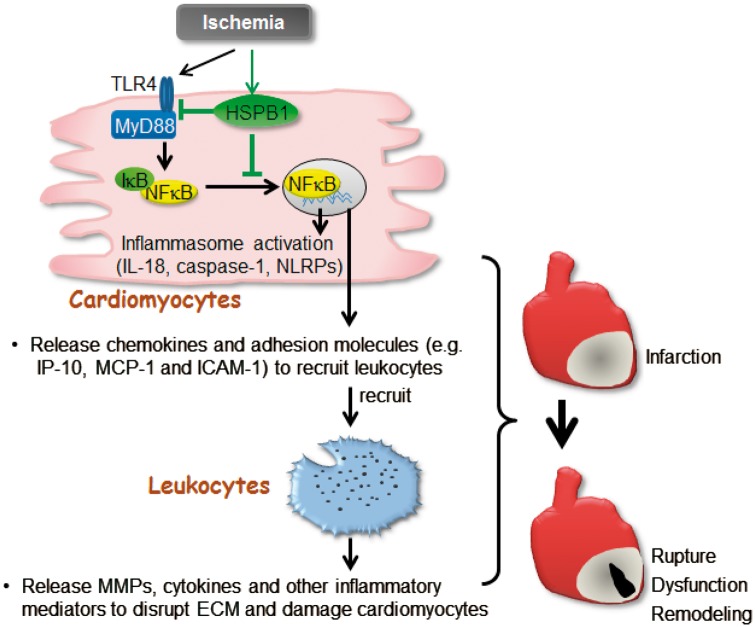
Figure 8.
Scheme represents that Cardiomyocyte-HSPB1 acts as a negatively regulator of NFκB-dependent inflammatory responses and plays essential role in wound healing after MI. In infarcted myocardium, cardiomyocyte TLR4/MyD88 was activated, and IκBα was phosphorylated and degraded, leading to NFκB nuclear translocation to stimulate the expression of inflammatory mediators including chemokines, cellular adhesion molecules, and the genes related to inflammasome activation. The chemokines and adhesion molecules from cardiomyocytes recruit leucocytes into infarcted myocardium to evoke inflammatory injury. HSPB1 was up-regulated in cardiomyocytes in response to MI, while knockout of cardiomyocyte-HSPB1 exaggerated the NFκB-dependent induction of chemokines for further leucocyte recruitment that leading to excessive inflammation, and ultimately caused cardiac rupture and worsened cardiac dysfunction following MI. cardiomyocyte HSPB1 is a negative regulator of NFκB proinflammatory signalling and plays an essential role in wound healing after MI.