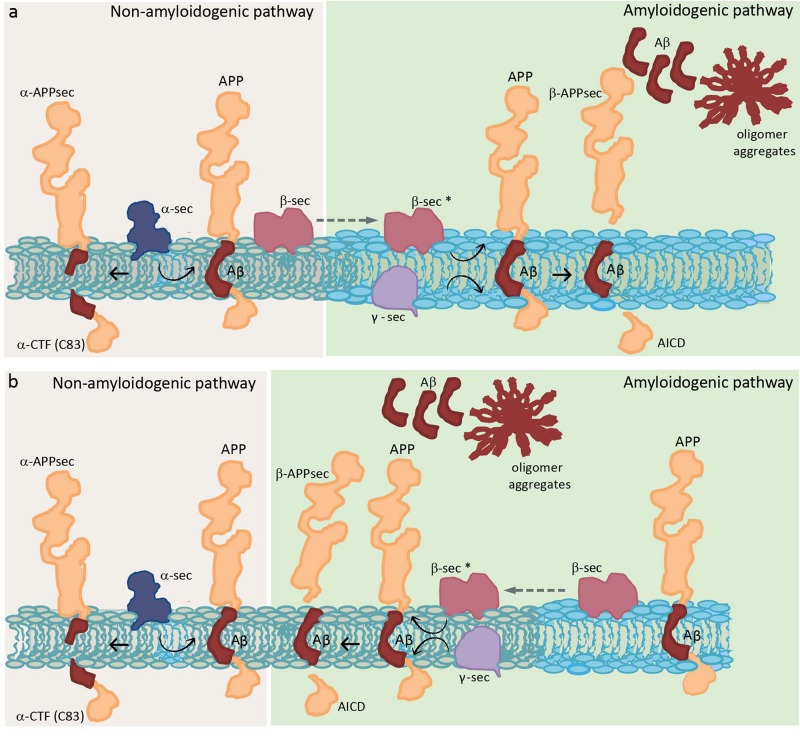
FIGURE 1.
Schematic diagram showing two distinct hypotheses of APP processing, which differ in the membrane location of the whole process. Two different colors are used to represent a raft domain and a liquid-disordered domain (light blue and gray, respectively). (a) Hypothesis where β sec is present in both raft and non-raft domains but needs to be in raft domains to be functional (represented as β sec*) (Ehehalt et al., 2003). (b) Hypothesis where β sec in raft domains corresponds to an inactive pool that needs to relocate to non-raft domains to be functional (Abad-Rodriguez et al., 2004). APP, amyloid precursor protein; α-CTF, C-terminal fragment obtained by α-secretase; α-APPsec, soluble N-terminal APP fragment obtained by α-secretase; Aβ, amyloid β peptide; β-APPsec, soluble N-terminal APP fragment obtained by β-secretase; AICD, APP intracellular domain obtained by the action of γ-secretase on β-CTF or C99 (intermediate peptide that is not shown and corresponds to Aβ plus AICD, obtained in the first step by the action of β-secretase); α-sec, α-secretase; β-sec, β-secretase; and γ-sec, γ-secretase.