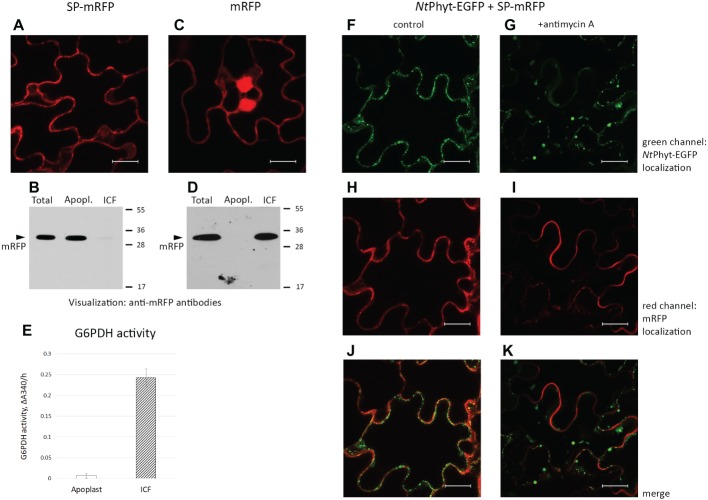
Figure 2.
Signal peptide-mRFP (SP-mRFP) as a soluble apoplastic protein marker. (A) Confocal fluorescence microscopy of N. benthamiana leaves producing SP-mRFP visualizes mRFP fluorescence at the cell borders. (B) Western blot analysis of mRFP (~30 kDa) distribution between the apoplastic (“Apopl.”) and intracellular (“ICF”) protein fractions. “Total” represents the leaf extract without fractionation. Anti-mRFP antibodies were used for protein detection. Positions of molecular weight protein markers are indicated on the right. (C) Fluorescence microscopy localization of free mRFP synthesized in N. benthamiana leaves. Bar in (A) and (C), 20 μm. (D) Western blot analysis demonstrating intracellular localization of free mRFP. Designations as in (B). (E) Determination of glucose 6-phosphate dehydrogenase (G6PDH) activity in the apoplastic (“Apoplast”) and intracellular (“ICF”) protein fractions. The activity was determined spectrophotometrically, by measuring change in absorbance at 340 nm/h (ΔΑ340/h). Average ± SD for three independent experiments with two replicates in each. (F–K) Using SP-mRFP as a marker to assess specificity of NtPhyt-EGFP internalization. Confocal fluorescence microscopy of N. benthamiana leaves co-producing NtPhyt-EGFP and SP-mRFP. The left column shows non-stressed leaves and the right column shows leaves treated with 10 μM antimycin A for 14 h. Bar, 20 μm.