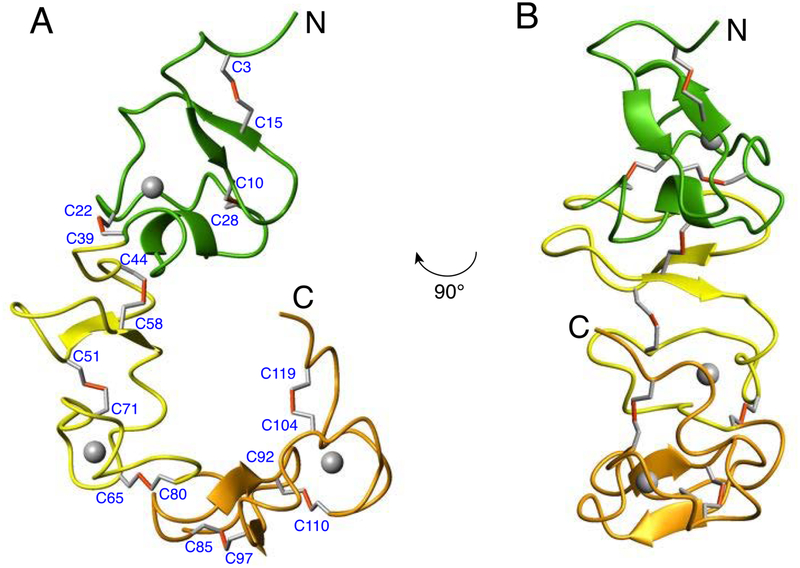
Figure 5.
(A) ribbon representation of the lowest energy structure of the VLDLR(2–4) fragment with CR2, CR3, and CR4 domains colored green, yellow, and orange, respectively; Ca2+ ions are shown as grey spheres. The structure depicts very short stretches of regular secondary structures and longer loop regions which are stabilized by extensive network of nine disulfide bonds. The pairing of cysteine residues are indicated with the disulfide bonds represented in red. (B) Another view of the ribbon representation of the VLDLR(2–4) structure highlighting the relative orientation of the antiparallel β-sheet in CR2 domain (green) which is almost 90° from CR3 (yellow).