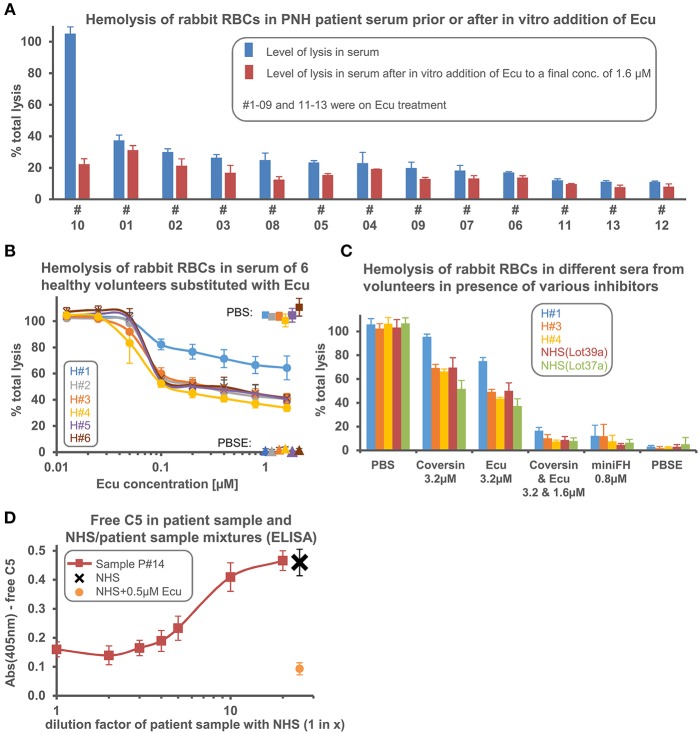
Figure 2.
Serum from different individuals exhibits different levels of residual terminal complement pathway activity in the presence of excess amounts of Eculizumab or Coversin. (A) Residual hemolysis was tested in sera of 13 PNH patients as in Figure 1A, but this time only one concentration of Eculizumab was added. Hemolysis was measured in serum or in serum that had been supplemented in vitro with Eculizumab to reach a final concentration of “in vitro added Eculizumab” of 1.6 μM (final serum content was 25%). #01–09 and #11–13 were on Eculizumab treatment; thus, serum from these patients already contained Eculizumab prior to the in vitro addition of extra Eculizumab. Patient #10 was not on Eculizumab treatment. (B,C) Residual lytic activity in the presence of Eculizumab or Coversin was tested in sera from six healthy volunteers and in commercially available pooled NHS sources (the two different lots mean independent pools) which are certified to possess full complement activities (average of 3 independent assays with SD is shown). (D) Determination of free C5 levels. In a sandwich ELISA, Eculizumab was used as a capture antibody and a polyclonal anti-C5 as detection antibody. Eculizumab adsorbed to the microtiter plate captured free C5 from NHS, but not when NHS was pre-mixed with 0.5 μM Eculizumab. The addition of 0.5 μM Eculizumab to NHS served as a reference since the C5 concentration in NHS is about 0.5 μM. No free C5 was captured from the patient sample or patient samples that had been diluted 1:1 (1-in-2) with NHS (average of 3 independent assays with SD shown). H#1–H#6, numbering of healthy volunteers enrolled in the study. Other abbreviations see legend to Figure 1.