Figure 2.
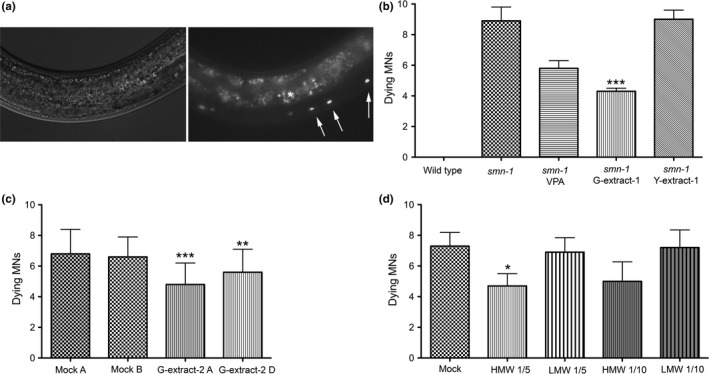
Kiwifruit effects on MN apoptotic death in transgenic C. elegans. Apoptotic death caused by smn‐1 knockdown is rescued by green kiwifruit extracts. (a) Accumulation of apoptotic‐related fluorescence in dying MNs in the ventral cord in a smn‐1(RNAi) transgenic animal (white arrows). Images were taken with visible light (left panel) and with epifluorescence (right panel), using Zeiss filter set 09 to distinguish it from intestinal autofluorescence (asterisk). Anterior is left, and ventral is down. (b‐d) Number of dying MNs accumulating apoptotic fluorescence in control and transgenic animals under different conditions. Bars represent the average number of dying neurons per animal, from at least two independent blind experiments; SEM or SD (in c) is shown. (b) smn‐1(RNAi) transgenic nematodes were treated with 1mM VPA, G‐extract‐1, and Y‐extract, diluted 1:10. The extract was omitted in the control (smn‐1). Wild‐type animals present no MN death. The number of animals observed is 53, 277, 100, 198, and 90, respectively. ***indicates significantly different from untreated animals (p < 0.001 with one‐way ANOVA). (c) Transgenic nematodes were treated with G‐extract‐2, at 1:10 dilution with heat‐killed (G‐extract‐2 D) or live (G‐extract‐2 A) bacteria and compared to untreated controls (mock). The number of animals observed is 76, 63, 77, and 45, respectively. *** and **indicate significantly different from relative untreated animals (p < 0.001 and p < 0.01, respectively, with one‐way ANOVA). (d) Transgenic nematodes were treated with high (>3 kDa, HMW) and low (<3 kDa, LMW) molecular weight fractions at 1:5 and 1:10 dilutions and compared to untreated controls (mock). The number of animals observed is 86, 81, 77, 91, and 82, respectively. *indicates significantly different from untreated animals (p < 0.05 with Unpaired t test)
