Abstract
Background
To assess the relationship between lung cancer and emphysema subtypes.
Objective
Airflow obstruction and emphysema predispose to lung cancer. Little is known, however, about the lung cancer risk associated with different emphysema phenotypes. We assessed the risk of lung cancer based on the presence, type and severity of emphysema, using visual assessment.
Methods
Seventy-two consecutive lung cancer cases were selected from a prospective cohort of 3,477 participants enrolled in the Clínica Universidad de Navarra’s lung cancer screening program. Each case was matched to three control subjects using age, sex, smoking history and body mass index as key variables. Visual assessment of emphysema and spirometry were performed. Logistic regression and interaction model analysis were used in order to investigate associations between lung cancer and emphysema subtypes.
Results
Airflow obstruction and visual emphysema were significantly associated with lung cancer (OR = 2.8, 95%CI: 1.6 to 5.2; OR = 5.9, 95%CI: 2.9 to 12.2; respectively). Emphysema severity and centrilobular subtype were associated with greater risk when adjusted for confounders (OR = 12.6, 95%CI: 1.6 to 99.9; OR = 34.3, 95%CI: 25.5 to 99.3, respectively). The risk of lung cancer decreases with the added presence of paraseptal emphysema (OR = 4.0, 95%CI: 3.6 to 34.9), losing this increased risk of lung cancer when it occurs alone (OR = 0.7, 95%CI: 0.5 to 2.6).
Conclusions
Visual scoring of emphysema predicts lung cancer risk. The centrilobular phenotype is associated with the greatest risk.
Introduction
Studies exploring the association between lung cancer and chronic obstructive pulmonary disease (COPD) have focused on airway obstruction (AO) or on self-reported chronic bronchitis or emphysema [1–4]. A recent analysis of the National Lung Screening Trial (ACRIN), reported a strong linear relationship between increasing severity of airflow obstruction and lung cancer risk, while several lung cancer screening cohorts have reported an association between emphysema and lung cancer.[5,6] In the latter, AO and the presence of emphysema on LDCT were associated with a two-to-threefold increase in lung cancer risk. After adjusting for confounders such as tobacco exposure, age, sex, and the presence of AO, emphysema is the strongest predictor of risk, even in non-smokers.[7–9] When comparing the impact of emphysema in the context of lung cancer screening in current, former and never smokers exposed to second hand smoke, the risk of lung cancer in never smokers with emphysema is similar to that in smokers (2.1% and 2.6%, respectively, p = 0.61), and 6-fold higher than in never smokers without emphysema.[10].
The association between emphysema and lung cancer risk has been significant primarily in studies in which visual assessment, as opposed to computer assessment, was performed.[5,6, 11–15] No study, however, has investigated the contribution of different emphysema phenotypes to lung cancer risk. Centrilobular emphysema (CLE) is associated with smoking and its distribution mimics that of lung cancer including upper lobe predominance.[16] We studied the relationship between lung cancer risk, AO and emphysema phenotype and severity using widely accepted visual scoring methods.
Materials and methods
Participants
Study subjects were enrolled in our lung cancer screening program between September 2000 and December 2016.[17] Inclusion criteria were age > 40 years, and a cumulative tobacco exposure of ≥ 10 pack-years. Patients with lung cancer symptoms, or a history of other cancers within 5 years were excluded from screening. The screening protocol was approved by the Research Ethics committee of Navarra University (project number 028/2012 mod 1, entitled “I-ELCAP”) and all subjects signed an informed consent prior to enrollment. Details of the screening protocol may be found in www.ielcap.org.
A total of 73 patients were diagnosed with lung cancer among 3,477 subjects enrolled in the screening program. One patient was excluded from the present study because imaging studies were not technically analyzable. Lung cancer was diagnosed on baseline or annual screenings in 30 and 42 participants respectively, with a mean of 40.7 months between the first visit and the cancer diagnosis. After adjusting for sex, age, pack-years, and smoking status, 216 individuals from the screened cohort were selected as controls with a 1:3 ratio of lung cancer cases to controls.
Pulmonary function tests
Pulmonary function tests (PFT) were performed with a flow spirometer (Vmax22; SensorMedics, Yorba Linda, CA) according to ATS guidelines.[18] Post-bronchodilator values were expressed as a percentage of the predicted value according to the European Community Lung Health Survey.[19] The presence and severity of AO was determined using criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD; forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ratio <70%) [20].
Low dose chest CT (LDCT)
Eleven cases and 19 controls included in this study underwent single-slice helical scanning (Somatom Plus 4; Siemens; Erlangen, Germany) at low-dose settings (140 kVp, 43 mAs) and 1.5 pitch with a collimation of 8 mm, providing. The rest of the cohort was scanned using a sixty-four slice multidetector CT scanner (Somatom Sensation 64, Somatom Definition, Siemens Healthcare, Erlangen, Germany) at a low-dose setting (120 kV tube voltage, 40 mAs tube current, 64x0.6 mm slice collimation, 0.5 s gantry rotation time, 1.4 pitch, 1 mm slice thickness, 1 mm reconstruction interval). Examinations were acquired with patients in the supine position, in cranio-caudal direction and at end-inspiration. Resulting images were reconstructed with a high convolution reconstruction algorithm (B60) and lung window [21].
Assessment of emphysema on LDCT
A pulmonologist (JG) visually scored the baseline LDCT for emphysema presence, type and severity, using validated criteria established by the Fleischner Society.[22] In general, pulmonary emphysema was classified into the following subtypes: centrilobular (CLE), panlobular (PLE) and paraseptal (PSE). There were no individuals with bullous or advanced destructive emphysema in our cohort. Only one subject was classified as PLE and was excluded from the analysis. For the purpose of data analysis, subjects were assigned to three categories: 1) No emphysema, 2) CLE and 3) PSE. Scoring procedures used a five-level semiquantitative scale based on criteria used in the National Emphysema Treatment Trial.[23] However, no division of the lung into different zones was undertaken. Severity was assessed using this scoring system throughout the whole lung.
For internal quality control, all 72 cases and 50 random controls were also independently reviewed by a radiologist (MC). Kappa statistics for the reading agreement between the pulmonologist (JG) and the radiologist was 0.87 (p<0.00001). Both readers (JG and MC) were blinded to lung cancer diagnosis when evaluating emphysema presence and severity.
Statistics
Statistical analysis was performed using the matched set of seventy-two cancer cases and 216 control subjects. Conditional logistic regression (SAS version 9.4; SAS Institute, Cary, NC) was used to assess whether %predicted FEV1 or FEV1/FVC, were risk factors for lung cancer. Lung function related variables were studied as continuous and ordinal variables, using the GOLD classification for FEV1: I: ≥80%; II: 50–80%; II: 30–50% and IV: <30%. Twelve patients were reclassified into a different GOLD group when using the post-bronchodilator FEV1. Additional analyses were performed adjusting for GOLD classification, visual emphysema and smoking status.
Results
Baseline characteristics of the 72 study patients with lung cancer and the 216 screened controls without lung cancer are shown in Table 1. As expected, there were no significant differences between subjects in average age and average pack-years of smoking. There were, however, more current smokers in the group of cases with cancer (73.6% vs 52.1%, p = 0.0014) and for this reason all analyses were adjusted by smoking status.
Table 1. Patient demographics.
| N = 287 | |||
|---|---|---|---|
| Demographic | Case Subjects (n = 72) | Control Subjects (n = 215) | p-value |
| Sex, No. (%) | 0.99 | ||
| Female | 12 (16.7) | 36 (16.7) | |
| Male | 60 (83.3) | 179 (83.3) | |
| Age, y | 0.83 | ||
| Mean (SD) | 63.8 (9) | 63.6 (8.8) | |
| Pack-years of smoking | 0.64 | ||
| Mean (SD) | 53.0 (25) | 51.5 (22.9) | |
| Smoking status, No. (%) | 0.0014 | ||
| Active smokers | 53 (73.6%) | 112 (52.1%) | |
| BMI | 0.46 | ||
| Mean (SD) | 26.7 (4.7) | 27.2 (3.9) | |
| Airflow obstruction, No. (%) | |||
| None | 33 (45.8) | 150 (69.8) | 0.0004 |
| GOLD I | 27 (37.5) | 36 (16.3) | |
| GOLD II | 9 (12.5) | 27 (12.6) | |
| GOLD III-IV | 3 (4.2) | 3 (1.4) | |
| Emphysema, No. (%) | 59 (81.9) | 90 (41.8) | <0.001 |
| CLE, No. (%) | 30 (50.8) | 9 (10) | <0.001 |
| PSE, No. (%) | 2 (3.4) | 31 (34.4) | |
| CLE and PSE, No. (%) | 27 (45.8) | 50 (55.5) | |
Definition of abbreviations: SD = Standard deviation; BMI = body mass index; GOLD = Global Initiative for Chronic Obstructive Lung Disease; CLE = Centrilobular; PSE = Paraseptal.
Adenocarcinoma (50%) was the most frequent type of lung cancer, followed by squamous cell carcinoma (20.8%). Other cell types included small cell carcinoma (6.9%), large cell carcinoma (9.7%), neuroendocrine tumors (5.6%), and mixed tumors (4.2%). Histology was unknown in two patients. Six patients had a second primary lung cancer identified on subsequent screening rounds. Staging based on clinical assessment or pathologic findings when available, was distributed as follows: 71% (51/72) in stage I, 8.3% (6/72) in stage II, 9.72% (7/72) in stage III and 11.11% (8/72) in stage IV.
Conditional logistic regression was used to determine whether airflow obstruction and the presence of radiographic emphysema are predictors of lung cancer risk independent of age, sex and smoking history (Table 2). Moreover, we performed an interaction model to try to clarify the relationship of the different emphysema subtypes and lung cancer (Table 3). Due to differences between the groups, all analyses were adjusted by smoking status.
Table 2. Multivariate conditional logistic results.
| Stratified on matching pairs* | Adjusted for emphysema* | Adjusted for airflow obstruction* | ||||||
|---|---|---|---|---|---|---|---|---|
| Case Subjects (n = 72) | Control Subjects (n = 215) | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Pulmonary function test | ||||||||
| Airflow obstruction | (0.0006) | (0.1244) | ||||||
| None | 33 | 150 | Ref. | Ref. | ||||
| Yes | 39 | 65 | 2.85 | 1.56–5.21 | 1.70 | 0.86–3.34 | ||
| (0.0020) | (0.1026) | |||||||
| None | 33 | 150 | Ref. | Ref. | ||||
| GOLD I | 27 | 35 | 3.77 | 1.85–7.68 | 2.50 | 1.13–5.51 | ||
| GOLD II | 9 | 27 | 1.65 | 0.68–3.96 | 0.89 | 0.34–2.33 | ||
| GOLD III-IV | 3 | 3 | 4.13 | 0.65–26.3 | 1.56 | 0.20–11.86 | ||
| Visual emphysema | ||||||||
| Emphysema | (<0.0001) | (<0.0001) | ||||||
| None | 13 | 125 | Ref. | Ref. | ||||
| Any | 59 | 90 | 5.94 | 2.90–12.17 | 5.44 | 2.61–11.35 | ||
| NETT classification | (<0.0001) | (<0.0001) | ||||||
| 0 | 13 | 125 | Ref. | Ref. | ||||
| 1 | 47 | 78 | 5.39 | 2.59–11.24 | 5.16 | 2.44–10.91 | ||
| 2 | 9 | 10 | 9.64 | 3.15–29.51 | 8.72 | 2.72–27.97 | ||
| 3 | 3 | 2 | 15.32 | 2.14–109.71 | 12.56 | 1.58–99.90 | ||
Definition of abbreviations: CI = confidence interval; SD = standard deviation; GOLD = Global Initiative for Chronic Obstructive Lung Disease; OR = odds ratio; NETT = National Emphysema Treatment Trial
* All adjusted by smoking status
Table 3. Multivariate conditional logistic results, emphysema types.
| Adjusted for airflow obstruction* | ||||
|---|---|---|---|---|
| Case Subjects (n = 72) | Control Subjects (n = 216) | OR | 95% CI | |
| Type of emphysema | (<0.0001) | |||
| None | 13 | 125 | Ref. | |
| CLE | 30 | 9 | 34.27 | 25.48–99.31 |
| CLE and PSE | 27 | 50 | 4.02 | 3.61–34.97 |
| PSE | 2 | 31 | 0.68 | 0.54–2.64 |
| (0.094) | ||||
| PSE by CLE Interaction | 27 | 21 | 0.17 | 0.03–1.35 |
Definition of abbreviations: CI = confidence interval; SD = standard deviation; GOLD = Global Initiative for Chronic Obstructive Lung Disease; OR = odds ratio; NETT = National Emphysema Treatment Trial; CLE = Centrilobular; PSE = Paraseptal
* All adjusted by smoking status
When FEV1 was analyzed as a continuous variable, the postbronchodilator percent predicted FEV1 (PFEV1%) was significantly associated with lung cancer risk (OR = 1.2, 95% CI: 1.0–1.4, p = 0.02). When spirometric severity by GOLD classification was used, only GOLD I remained significantly associated with lung cancer risk (Table 4).
Table 4. Univariate conditional logistic regression results.
| Pulmonary function test | |||||
|---|---|---|---|---|---|
| Measure | Case Subjects (n = 72) | Control Subjects (n = 215) | OR | 95% CI | p-value |
| PFEV1, % predicted | 0.02 | ||||
| Mean (SD) | 87.9 (19) | 94.2 (19.5) | 1.2 | (1.0–1.4) | |
| 81+ | 51 (70.8%) | 161 (74.9%) | Ref. | ||
| 51–80 | 17 (23.6%) | 38 (17.7%) | 1.5 | (0.7–2.9) | |
| 31–50 | 3 (4.2%) | 5 (2.3%) | 2.1 | (0.4–11) | |
| ≤ 30 | 1 (1.4%) | 11 (5.1%) | 0.3 | (0.04–2.3) | |
| PFEV1/FVC | 0.01 | ||||
| Mean (SD) | 66.6 (10.3) | 70.6 (10.3) | 1.6 | (1.2–2.0) | |
| 71+ | 30 (41.7%) | 136 (63.3%) | Ref. | ||
| 61–70 | 24 (33.3%) | 40 (18.6%) | 3.2 | (1.6–6.5) | |
| 51–60 | 10 (13.9%) | 13 (6%) | 3.9 | (1.5–10.2) | |
| ≤ 50 | 8 (11.1%) | 26 (12.1%) | 1.6 | (0.6–4.0) | |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity; P = postbronchodilator
The presence of visually determined emphysema on the baseline chest CT was significantly associated with an increased risk of lung cancer (OR = 5.9, 95% CI: 2.9–12.7; p<0.0001). Moreover, this risk was related to the severity of visual emphysema quantified according to the NETT classification: for NETT values of 1 to 3, the OR was 5.4 (95% CI: 2.6–11.2), 9.6 (95% CI: 3.2–29.5) and 15.3 (95% CI: 2.1–109.7), respectively.
In the second logistic regression, the relationship between airflow obstruction and the risk of lung cancer was no longer significant after adjusting for visual emphysema. However, visual emphysema remained strongly associated with lung cancer risk after adjusting for COPD GOLD classification (OR, 5.4; 95% CI, 2.6–11.4; p<0.0001). Additionally, the risk of lung cancer was higher as the emphysema severity increased according to the NETT classification [OR 5.2 (95% CI: 2.4–10.9), 8.7 (95% CI: 2.7–27.9) and 12.5 (95% CI: 1.6–99.9), for categories 1, 2 and 3 respectively].
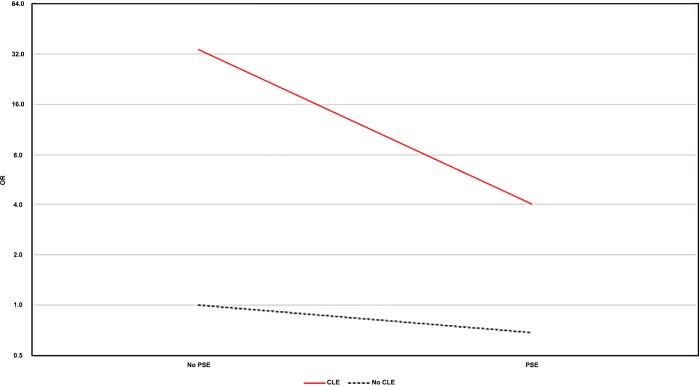
To explore the relationship between emphysema subtypes and lung cancer risk, patients were grouped according to the emphysema subtype. Afterwards, an interaction model was completed to correctly assess this relationship. In comparison to patients without emphysema, the risk of lung cancer in patients with CLE alone was 34-fold greater (OR = 34.27, 95% CI: 25.48–99.31; p<0.000), whereas in patients with concomitant PSE the OR was 4.02 (95% CI: 3.61–34.97). Surprisingly, PSE alone did not show a clear risk of lung cancer with an OR of 0.68 (95% CI: 0.54–2.64; p<0.635). This can be seen better through the Fig 1.
Fig 1. Odds ratios (OR) of the differents emphysema subtypes and lung cancer in the interaction model.
This figure represents the ORs of the different emphysema subtypes with lung cancer. As it is shown, CLE (centrilobular) alone has the highest risk to develop lung cancer which dramatically decreases with the added presence of PSE (paraseptal). Surprisingly, PSE alone is not giving a differential risk of lung cancer.
Associated data
Data is available on Figshare (https://doi.org/10.6084/m9.figshare.8046461.v1).
Discussion
Our study contributes to existing evidence linking emphysema with lung cancer risk, and goes one step further by refining our knowledge of the risk associated with emphysema subtypes. [5,6,13,14] The finding that smokers with CLE are at greatest risk in comparison with those with PSE seems to point to a phenotypic predisposition to lung cancer related to emphysema distribution. We also confirmed previous reports suggesting that airflow obstruction is only associated with a higher risk for lung cancer on univariate analysis, but not after adjusting for the presence of emphysema.[3,4,24] This suggests that most of the lung cancer risk attributed to COPD may be due to the presence of emphysema.[5,6] Since many patients with emphysema do not have AO, it is important to emphasize this finding, just as current guidelines make therapeutic distinctions based on COPD phenotypes recommending different treatments for the emphysema and chronic bronchitic phenotypes.[25] A recent study by Carr et al., has also refined our understanding of the lung cancer risk associated with COPD by focusing on important differences in the features of patients with COPD that condition lung cancer risk.[26] In their study, COPD and its severity, respiratory exacerbations, and of course visual emphysema were independent predictors of lung cancer. As our understanding of the associations between COPD, emphysema and lung cancer grows, so does the conviction that risk is linked to specific phenotypes.
To the best of our knowledge, no previous studies have assessed the association between lung cancer and emphysema stratified by emphysema subtype. That CLE is associated with an increased risk for lung cancer makes biological sense since it is the subtype of emphysema more closely associated with smoking.[27,16] Moreover, CLE occurs more frequent in the upper lobes and in the right lung, just as cancer does.[16,28] The association between cigarette smoking and CLE has been attributed to systemic chronic inflammation and lung repair mechanisms unique to the exposure and perhaps a genetic predisposition. Patients with CLE have higher white blood cell counts [16] and a unique protease-antiprotease balance, characterized by a higher expression of matrix metalloproteinase 9 (MMP9) and transforming growth factor beta 1(TGFB1).[29] The former has been linked to both emphysema [30,31] and cancer [32,33]. In contrast to CLE, patients with PSE are often free of respiratory symptoms and are generally spared of the functional decline typically seen in patients with CLE.[16] PSE occurs in the absence of tobacco exposure and may depend on age and genetic susceptibility. Furthermore, PSE has been associated with the inhibitor of metalloproteinases 2 (TIMP2) and tumor necrosis factor.[29] The former is a tyrosine kinase receptor inhibitor of growth factor-mediated proliferation in both normal and tumor cells [34]. The activation of TIMP2 in tumor cells inhibits tumor cell growth in vivo and suppresses epithelial-to-mesenchymal transition (EMT), which is associated with tumor characteristics of aggressiveness and metastatic potential [35]. Therefore, it is likely that emphysema subtypes represent different disease phenotypes with implications beyond characteristic imaging findings, representing different genetic susceptibilities, molecular mechanisms, exposure to toxins, and clinical manifestations including risk of lung cancer.
Our study also found a linear trend between emphysema severity as measured by the NETT classification and lung cancer. Other studies have found an all-or-none effect.[5–7] For example, Wilson et al. found that lung cancer risk was greater for individuals with mild emphysema, followed by those with moderate or severe emphysema, and those with traces of emphysema.[6] Similarly, Li et al [7] found that lung cancer risk did not increase with emphysema severity (OR = 3.33; 95% CI: 2.30–4.82, and OR = 3.80; 95% CI: 2.78–5.19, for ≥10% and ≥5% emphysema, respectively). In a lung cancer screening cohort of 9,047 subjects from New York [36], the presence of emphysema, independently of its severity, predicted death from lung cancer (OR = 3.2; 95% CI: 1.5–6.7).
Limitations
Our study is limited by group differences insofar as there were more active smokers in the group with lung cancer compared to controls. However, we made every attempt to correct for this potential bias by adjusting our analysis for pack-years smoked (matching criterion) and smoking status. Another limitation may be the predominance of male patients in our study (83% in cases and controls) as compared to other studies (Wilson et al.: 51.4%, De Torres et al.: 74%) [5,6]. This is important as women have been shown to demonstrate less radiographic evidence of emphysema than men.
Conclusions
Our study shows that visually assessed CLE, and not PSE, is strongly associated with an increased risk of lung cancer. We believe our findings refine our current understanding of the risks associated with emphysema and contribute to a growing body of knowledge suggesting that emphysema subtypes represent specific phenotypes conditioned by genetic susceptibility, toxin exposure, molecular mechanisms and ultimately divergent clinical outcomes, which may have implications for lung cancer screening and follow up.
Data Availability
All relevant data are available within the Figshare Repository at https://doi.org/10.6084/m9.figshare.8046461.v1.
Funding Statement
This work was supported in part by a grant [RD12/0036/0062 and RD12/0036/0040] from Red Tematica de Investigacion Cooperativa en Cancer, Instituto de Salud Carlos III, the Spanish Ministry of Economy and Competitiveness and European Regional Development Fund “Una manera de hacer Europa.” It was also supported by grants PI04/2404, PI07/0792, PI10/01652, and PI11/01626 from the Instituto de Salud Carlos III, Ministry of Economy and Competitiveness, Government of Spain. The funder provided support in the form of salaries for authors [JJZ, JPd-T, DY, CH, AR]. The specific roles of these authors are articulated in the author contributions section. Funding agencies were not involved in study design, data collection nor analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zulueta JJ. Emphysema and Lung Cancer. More than a coincidence. Ann Am Thorac Soc 2015;12(8):1120–1. 10.1513/AnnalsATS.201506-360ED [DOI] [PubMed] [Google Scholar]
- 2.Samet JM, Humble CG, Pathak DR. Personal and Family History of Respiratory Disease and Lung Cancer Risk. Am Rev Respir Dis 1986. September 1;134(3):466–70. 10.1164/arrd.1986.134.3.466 [DOI] [PubMed] [Google Scholar]
- 3.Skillrud DM, Offord KP, Miller RD. Higher Risk of Lung Cancer in Chronic Obstructive Pulmonary DiseaseA Prospective, Matched, Controlled Study. Ann Intern Med 1986. October 1;105(4):503–7. 10.7326/0003-4819-105-4-503 [DOI] [PubMed] [Google Scholar]
- 4.Tockman MS, Anthonisen NR, Wright EC, Donithab MG. Airways Obstruction and the Risk for Lung Cancer. Ann Intern Med 1987. April 1;106(4):512–8. 10.7326/0003-4819-106-4-512 [DOI] [PubMed] [Google Scholar]
- 5.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 2007;132(6):1932–8. 10.1378/chest.07-1490 [DOI] [PubMed] [Google Scholar]
- 6.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008;178(7):738–44. 10.1164/rccm.200803-435OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Swensen SJ, Karabekmez LG, Marks RS, Stoddard SM, Jiang R, et al. Effect of emphysema on lung cancer risk in smokers: a computed tomography-based assessment. Cancer Prev Res 2011. January;4(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zulueta JJ. Emphysema Scores Predict Death From COPD and Lung Cancer. Chest 2012;141(5):1216 10.1378/chest.11-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med 2007;176(3):285–90. 10.1164/rccm.200612-1792OC [DOI] [PubMed] [Google Scholar]
- 10.Henschke CI, Yip R, Boffetta P, Markowitz S, Miller A, Hanaoka T, et al. CT screening for lung cancer: Importance of emphysema for never smokers and smokers. Lung Cancer 2015;88(1):42–7. 10.1016/j.lungcan.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 11.Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: A systematic review and meta-analysis. Lung Cancer 2012;77(1):58–63. 10.1016/j.lungcan.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 12.Kishi K, Gurney JW, Schroeder DR, Scanlon PD, Swensen SJ, Jett JR. The correlation of emphysema or airway obstruction with the risk of lung cancer: a matched case-controlled study. Eur Respir J 2002. June;19(6):1093–8. [DOI] [PubMed] [Google Scholar]
- 13.Maldonado F, Bartholmai BJ, Swensen SJ, Midthun DE, Decker P a, Jett JR. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest 2010. December;138(6):1295–302. 10.1378/chest.09-2567 [DOI] [PubMed] [Google Scholar]
- 14.Wilson DO, Leader JK, Fuhrman CR, Reilly JJ, Sciurba FC, Weissfeld JL. Quantitative Computed Tomography Analysis, Airflow Obstruction, and Lung Cancer in the Pittsburgh Lung Screening Study. J Thorac Oncol 2011;6(7):1200–5. 10.1097/JTO.0b013e318219aa93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aamli Gagnat A, Gjerdevik M, Gallefoss F, Coxson HO, Gulsvik A, Bakke P. Incidence of non-pulmonary cancer and lung cancer by amount of emphysema and airway wall thickness: a community-based cohort. Eur Respir J 2017. May 11;49(5). [DOI] [PubMed] [Google Scholar]
- 16.Smith BM, Austin JH, Newell JD Jr, Souza BM, Rozenshtein A, Hoffman EA, Ahmed F, Barr RG. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med 2015;127(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henschke C.I, Yankelevitz D.F, Libby DM., Pasmantier M.W., Smith J.P., Miettinem O. S.. Survival of patients with stage I lung cancer detected on CT screening. N. Engl. J. Med 2006;1763–71. 10.1056/NEJMoa060476 [DOI] [PubMed] [Google Scholar]
- 18.Celli BR, Macnee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS / ERS position paper. Eur Resp J 2004;932–46. [DOI] [PubMed] [Google Scholar]
- 19.Roca J, Burgos F, Sunyer J, Saez M, Chinn S, Anto JM, et al. References values for forced spirometry. Group of the European Community Respiratory Health Survey. Eur Respir J 1998. June 1;11(6):1354 LP-1362. [DOI] [PubMed] [Google Scholar]
- 20.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DMG, López Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agustí A. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557–582. 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 21.Henschke CI, Mccauley DI, Yankelevitz DF, Naidich DP, Mcguinness G, Miettinen OS, et al. E a rly Lung Cancer Action Project: overall design and findings from baseline screening. Cancer 1999;354:99–105. [DOI] [PubMed] [Google Scholar]
- 22.Society F, Austin JHM, Hogg JC, Grenier PA, Barr RG, Colby T V, Galvin JR, Gevenois PA, Coxson HO, Hoffman EA, Newell JD. Chronic Obstructive Pulmonary Disease: A Statement of the Fleishner Society. Radiology 2015;277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Emphysema Treatment Trial Research Group. Patients at High Risk of Death after Lung-Volume–Reduction Surgery. N Engl J Med 2001;345:1075–1083. 10.1056/NEJMoa11798 [DOI] [PubMed] [Google Scholar]
- 24.Wasswa-Kintu S, Gan WQ, Man SFP, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax 2005;60(7):570–5. 10.1136/thx.2004.037135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Antonio Quintano J, et al. Spanish Guideline for COPD (GesEPOC). Update 2014. Arch Bronconeumol. 2014;50(S1):1–16. [DOI] [PubMed] [Google Scholar]
- 26.Carr LL, Jacobson S, Lynch DA, Foreman MG, Flenaugh EL, Hersh CP, et al. Features of Chronic Obstructive Pulmonary Disease as Predictors of Lung Cancer. Chest 2018; S0012-3692(18)30254-X [Google Scholar]
- 27.Copley SJ, Wells AU, Müller NL, Rubens MB, Hollings NP, Cleverley JR, et al. Thin-Section CT in Obstructive Pulmonary Disease: Discriminatory Value. Radiology 2002. June 1;223(3):812–9. 10.1148/radiol.2233010760 [DOI] [PubMed] [Google Scholar]
- 28.Byers TE, Vena JE, Rzepka TF. Predilection of lung cancer for the upper lobes: An epidemiologic inquiry. J Natl Cancer Inst 1984;72(6):1271–5. [PubMed] [Google Scholar]
- 29.Kukkonen MK, Tiili E, Vehmas T, Oksa P, Piirilä P, Hirvonen A. Association of genes of protease-antiprotease balance pathway to lung function and emphysema subtypes. BMC Pulm Med 2013;13(1). 10.1186/1471-2466-13-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foronjy R, Nkyimbeng T, Wallace A, Thankachen J, Okada Y, Lemaitre V, et al. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am J Physiol Lung Cell Mol Physiol 2008;294(6):L1149–57. 10.1152/ajplung.00481.2007 [DOI] [PubMed] [Google Scholar]
- 31.Vignola AM, Riccobono L, Mirabella A, Profita M, Chanez P, Bellia V, et al. Sputum Metalloproteinase-9/Tissue Inhibitor of Metalloproteinase-1 Ratio Correlates with Airflow Obstruction in Asthma and Chronic Bronchitis. Am J Respir Crit Care Med. 1998. December 1;158(6):1945–50. 10.1164/ajrccm.158.6.9803014 [DOI] [PubMed] [Google Scholar]
- 32.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci. 2006;103(33):12493–8. 10.1073/pnas.0601807103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Wu JZ, Zhang JY, Xue J, Ma R, Cao HX, et al. Detection of circulating vascular endothelial growth factor and matrix metalloproteinase-9 in non-small cell lung cancer using Luminex multiplex technology. Oncol Lett. 2014;7(2):499–506. 10.3892/ol.2013.1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remillard TC, Bratslavsky G, Jensen-Taubman S, Stetler-Stevenson WG, Bourboulia D. Molecular mechanisms of tissue inhibitor of metalloproteinase 2 in the tumor microenvironment. Mol Cell Ther. 2014;2(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourboulia D, Han H, Jensen-Taubman S. TIMP-2 modulates cancer cell transcriptional profile and enhances E-cadherin/beta-catenin complex expression in A549 lung cancer cells. Oncotarget. 2013;4(1):163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zulueta JJ, Wisnivesky JP, Henschke CI, Yip R, Farooqi AO, McCauley DI, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141(5):1216–23. 10.1378/chest.11-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available within the Figshare Repository at https://doi.org/10.6084/m9.figshare.8046461.v1.