Abstract
Hepatic inflammatory pseudotumors (IPTs) are rare lesions that mimic malignancy clinically, radiologically, and pathologically. The pathophysiology is unknown, and no criteria exist for diagnosis. This series includes 3 cases: 1 patient had recent biliary drainage with bile duct stent placement, and the other 2 patients had hepatic abscess formation before IPT development, which further supports that hepatic IPTs develop in patients with underlying triggers of liver inflammation and injury, including infections and/or bile leakage into the parenchyma. All 3 patients were successfully treated with antibiotics, sparing them surgical intervention. Follow-up showed complete resolution, and none developed recurrences or malignancies.
Introduction
Inflammatory pseudotumor (IPT) of the liver is a rare benign lesion that frequently resolves spontaneously.1 This lesion, however, may clinically and radiologically mimic a malignant liver tumor, placing it in the differential diagnosis in patients with hepatic space-occupying lesions.2–5 Given its rarity, more data are needed to help determine which clinical settings warrant increased suspicion for this diagnosis and to develop criteria for diagnosis and classification. This entity remains a diagnosis of exclusion after ruling out hepatocellular carcinoma, cholangiocarcinoma, metastatic tumor, inflammatory myofibroblastic tumors, follicular dendritic cell tumors, or inflammatory type of angiomyolipoma. Here, we report 3 recent cases of hepatic IPT. These patients all had 1 feature in common—manipulation of the biliary tree or liver before development of the lesion. One patient underwent endoscopic retrograde cholangiopancreatography with bile duct stent placement. Histology of the liver mass identified gram-negative bacterial organisms. The remaining 2 patients both had a history of liver abscess formation before the development of IPT. This case series highlights that an infectious process and/or bile leaking into the hepatic parenchyma can lead to hepatic IPT development.
Case Report
Patient 1
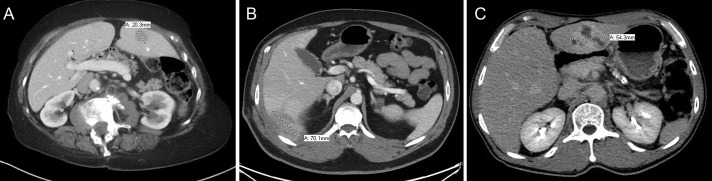
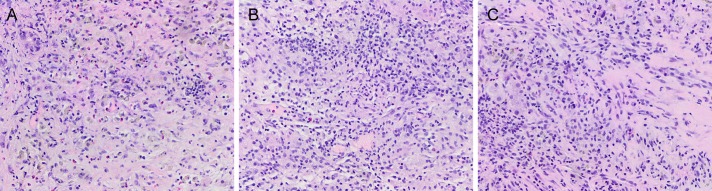
An 80-year-old woman with a history of colon and ovarian cancer was admitted for painless obstructive jaundice secondary to a possible periampullary mass and elevated CA19-9, features which were concerning for malignancy. Biopsy of the periampullary mass did not show malignancy, and the patient was treated with transhepatic biliary drainage and subsequent common bile duct stent placement through the percutaneous transhepatic approach to relieve her jaundice. One month later, abdominal computed tomography (CT) revealed small hypodense areas in the liver with the largest measuring 2 cm, concerning for metastasis (Figure 1). Laboratory tests revealed a normal complete blood count and hepatic enzymes and an elevated alkaline phosphatase level. Ultrasound-guided fine-needle aspiration and core biopsy of liver mass showed mixed inflammation with fibrous reaction and prominent cholestatic changes, consistent with IPT (Figure 2). Gram and Steiner stains were positive for bacterial organisms, most consistent with gram-negative rods, whereas ALK-1, IgG4, and in situ hybridization for Epstein-Barr virus (EBER) showed negative staining. Two months later, repeat CT imaging indicated interval-increased size of the intrahepatic collection. She was treated with amoxicillin-clavulanate for 3 months. After treatment, the patient's CA19-9 normalized, and follow-up CT imaging 6 months later demonstrated that her hepatic lesions had completely resolved.
Figure 1.
Abdominal computed tomographic image comparison for (A) patient 1, (B) patient 2, and (C) patient 3.
Figure 2.
Pathological findings show fibrous stroma with mixed inflammatory cell infiltration, consistent with inflammatory pseudotumor in (A) patient 1, (B) patient 2, and (C) patient 3. H&E stain (40× magnification).
Patient 2
A 49-year-old man with a history of perforated appendicitis and multifocal intra-abdominal abscesses had an abdominal CT, which revealed a 3.1-cm hypodense liver mass. Three months later, the patient was admitted with intermittent right upper quadrant pain, fever, and nausea. Magnetic resonance imaging showed that the liver mass had increased to 7.5 cm in size and appeared to extend outside of the liver involving adjacent tissues, suggesting cholangiocarcinoma or metastasis (Figure 1). Laboratory tests revealed mild anemia, no leukocytosis, and normal hepatic enzymes. However, at the time of his ultrasound-guided liver biopsy, purulent fluid was noted at the drain site, and culture identified Citrobacter koseri. He was started on ciprofloxacin (for 2 weeks). Follow-up CT imaging 2 weeks later showed resolution of the liver abscess with persistent adjacent hypoenhancement measuring approximately 7 cm, which was suspicious for intrahepatic cholangiocarcinoma. A liver core biopsy demonstrated IPT rather than malignancy (Figure 2). Immunostains showed focal positivity for smooth muscle actin and IgG4 but were negative for CD34, ALK-1, desmin, and EBER. Repeat imaging 7 months later revealed complete resolution.
Patient 3
A 59-year-old man with a previous history of pancreatic cholangiocarcinoma that was removed by a Whipple procedure 28 years ago presented with worsening right upper quadrant abdominal pain. Of note, he also had been diagnosed with a liver abscess 1 year before his current presentation. Abdominal CT revealed multiple hypodense hepatic lesions, which coalesced to measure up to 8 cm in size and were concerning for recurrent cholangiocarcinoma (Figure 1). Laboratory tests revealed leukocytosis, normal hepatic enzymes, and unremarkable CA19-9. Liver fine-needle aspiration and core biopsies showed mixed inflammation with fibrous reaction and focal abscess formation with necrosis, consistent with IPT (Figure 2). Immunostains showed occasional IgG4-positive plasma cells and were negative for acid-fast bacilli, GMS, ALK-1, and EBER. The patient's abscess was drained, and he was started on levofloxacin and metronidazole (for 2 months). Two months later, a follow-up CT showed complete resolution of the hepatic mass-like lesion.
Discussion
Hepatic IPTs are defined by the WHO classification as benign, non-neoplastic, nonmetastasizing masses characterized by the presence of myofibroblastic spindle cells, infiltrated plasma cells, and mixed inflammatory cells without cellular anaplasias or atypical mitoses.6,7 The lesions are well known to have extensive variability in histologic features, and no uniform criteria have been recommended for diagnosis and classification.1,8
The etiology of hepatic IPT remains largely unknown, and it has been associated with infection and inflammatory conditions including autoimmune diseases such as primary sclerosing cholangitis.9–11 Histological classification of hepatic IPT has not been well established. Balabaud et al conducted a study of 145 cases of liver IPTs from the Armed Forces Institute of Pathology and identified 5 histologic subgroups: a plasma cell–rich subgroup with aggregates of or diffuse lymphocytes, a mixed inflammatory cell subgroup, a granulomatous subgroup, a granulomatous subgroup with eosinophils, and a predominantly purulent subgroup.1 Bacterial organisms were identified in our first case by Steiner staining. Although 2 of the cases showed focally increased IgG4-positive plasma cells, these did not meet the currently accepted diagnostic criteria because too few of plasma cells were observed in the liver tissue and none of the patients had elevated levels of IgG4 in the serum.12,13 In summary, IPT may mimic malignant tumors in terms of clinical presentation, radiological imaging features, and histopathological findings14,15 and often results in unnecessary, invasive procedures. Both clinicians and pathologists should consider this differential in patients with hepatic mass lesions, especially if the patient has a clinical history indicating recent manipulation of the biliary tree and/or hepatic parenchyma that might result in bile extravasation and/or infection.
Disclosures
Author contributions: HL Stevenson designed the report and is the article guarantor. J. Zhao, K. Olino, LE Low, S. Qiu, and HL Stevenson collected the patient's clinical data. J. Zhao and HL Stevenson analyzed the data and wrote the manuscript.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Balabaud C, Bioulac-Sage P, Goodman ZD, Makhlouf HR. Inflammatory pseudotumor of the liver: A rare but distinct tumor-like lesion. Gastroenterol Hepatol (NY). 2012;8(9):633–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Park JY, Choi MS, Lim YS, et al. Clinical features, image findings, and prognosis of inflammatory pseudotumor of the liver: A multicenter experience of 45 cases. Gut Liver. 2014;8(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kai K, Matsuyama S, Ohtsuka T, Kitahara K, Mori D, Miyazaki K. Multiple inflammatory pseudotumor of the liver, mimicking cholangiocarcinoma with tumor embolus in the hepatic vein: Report of a case. Surg Today. 2007;37(6):530–3. [DOI] [PubMed] [Google Scholar]
- 4.Rosa B, Moutinho-Ribeiro P, Pereira JM, et al. Ghost tumor: An inflammatory pseudotumor of the liver. Gastroenterol Hepatol (NY). 2012;8(9):630–3. [PMC free article] [PubMed] [Google Scholar]
- 5.Tsou YK, Lin CJ, Liu NJ, Lin CC, Lin CH, Lin SM. Inflammatory pseudotumor of the liver: Report of eight cases, including three unusual cases, and a literature review. J Gastroenterol Hepatol. 2007;22(12):2143–7. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Zhu J, Biskup E, Cai F, Li A. Inflammatory pseudotumors of the liver: Experience of 114 cases. Tumour Biol. 2015;36(7):5143–8. [DOI] [PubMed] [Google Scholar]
- 7.Milias K, Madhavan KK, Bellamy C, Garden OJ, Parks RW. Inflammatory pseudotumors of the liver: Experience of a specialist surgical unit. J Gastroenterol Hepatol. 2009;24(9):1562–6. [DOI] [PubMed] [Google Scholar]
- 8.Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: Where are we now? J Clin Pathol. 2008;61(4):428–37. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Lu H, Ji H, Li Y. Inflammatory pseudotumor of the liver: A case report and literature review. Intractable Rare Dis Res. 2015;4(3):155–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosman F, Carneiro F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System. 4th ed International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- 11.Calomeni GD, Ataide EB, Machado RR, Escanhoela CA, Costa LB, Boin IF. Hepatic inflammatory pseudotumor: A case series. Int J Surg case Rep. 2013;4(3):308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umehara H, Okazaki K, Nakamura T, et al. Current approach to the diagnosis of IgG4-related disease: Combination of comprehensive diagnostic and organ-specific criteria. Mod Rheumatol. 2017;27(3):381–91. [DOI] [PubMed] [Google Scholar]
- 13.Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688–99. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo Y, Sato M, Shibata T, et al. Inflammatory pseudotumor of the liver diagnosed as metastatic liver tumor in a patient with a gastrointestinal stromal tumor of the rectum: Report of a case. World J Surg Oncol. 2014;12:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang SD, Scali EP, Abrahams Z, Tha S, Yoshida EM. Inflammatory pseudotumor of the liver: A rare case of recurrence following surgical resection. J Radiol Case Rep. 2014;8(3):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]