Fig. 9. Potential mechanisms of Nrf2-dependent sulforaphane action in lung protection.
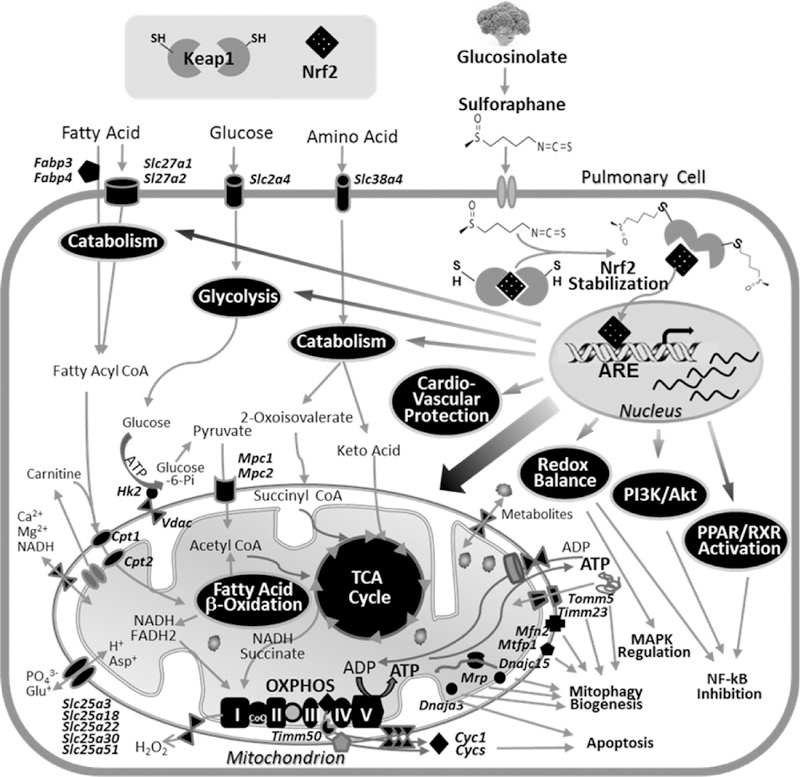
Dietary sulforaphane is known to stabilize cytoplasmic Nrf2 through Keap1 binding to inhibit Nrf2 ubiquitination for its proteasomal degradation. Stabilized Nrf2 may exert transcriptional activation of antioxidant response element (ARE)-responsive genes involved in redox regulation but also in fatty acid, glucose, and amino acid catabolism including enzymes and cellular transporters. Transcription activation then results in provision of metabolic substrates for mitochondrial machinery, tricarboxylic acid (TCA) cycle, fatty acid β-oxidation (FAO), and oxidative phosphorylation (OXPHOS) complex as well as mitochondrial symporters and transporters. Nrf2 may also directly upregulate ARE-bearing genes that encode these mitochondrial apparatus and mitochondrial quality control processes (i.e., mitophagy, biogenesis). Enhanced cyctochrome C (Cyc1) from OXPHOS is known to cause apoptosis, and ARE-responsive vasodilation and cardioprotective genes may protect pulmonary hypertension and vascular remodeling. Overall, sulforaphane-Nrf2 responses enrich mitochondrial bioenergetics to produce ATP on cellular demand but also facilitate other mitochondrial functions for cellular homeostasis against oxidative stress. Pathway analyses indicated that these Nrf2-dependently regulated lung genes are associated with pathways including peroxisome proliferator-activated receptor/retinoid X receptor (PPAR/RXR) activation, phosphatidylinositol 3- kinase (PI3K)/Akt signaling, which may modulate NF-κB and MAPK signaling cascade. Proteins and cellular processes predicted to be modulated by SFN-Nrf2 axis are highlighted in black.
