Figure 2: Overall structure of the hERG K+ channel.
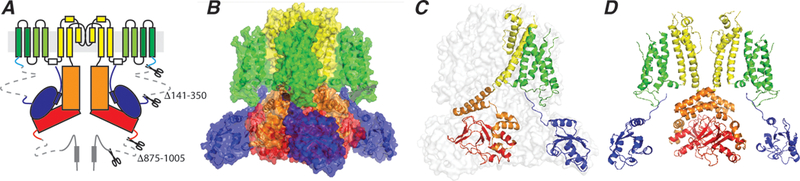
A. Topology of the hERG K+ channel showing two of the four subunits that constitute the tetrameric channel. The full-length monomer is 1159 residues. To generate a biochemically stable channel, residues 141–350 in the N-terminus and residues 875–1005 in the C-terminus were deleted.
B. The channel structure shown in surface representation, colour coded according to the scheme shown in panel A.
C. hERG structure shown in transparent surface representation with one subunit shown in cartoon representation colour coded by domains as shown in panel A. Note that the voltage sensor of each subunit interacts with the pore domain within its own subunit (in contrast to the domain swapped structure in classical voltage-gated ion channels).
D. Two opposing subunits are shown to highlight the open intracellular activation gate and the “domain swapped” architecture within the cytoplasmic C-terminal domains.
