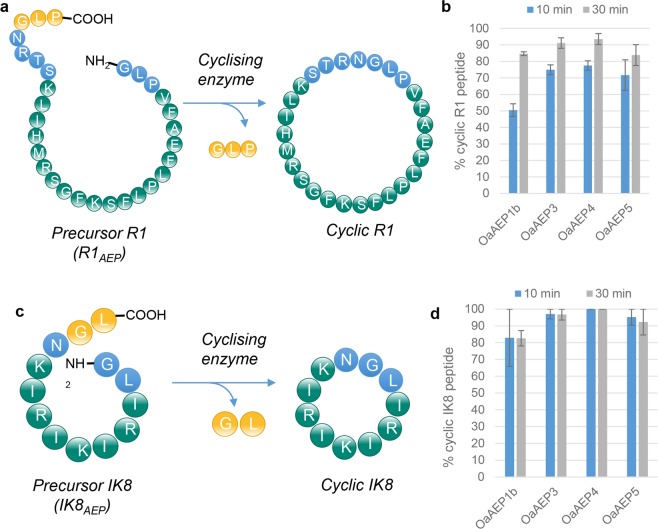
Figure 1.
Recombinant enzymes rapidly and efficiently cyclise non-native substrates. Recombinant AEPs were incubated with the non-native substrates (a) R1AEP or (c) IK8AEP peptide (each at 280 µM) and the products were assessed by MALDI-MS. The proportion of cyclic product was determined relative to all peaks attributed to the processed or unprocessed (b) R1 peptide or (d) IK8c peptide. Enzyme concentrations OaAEP1b 0.528 µM, OaAEP3 0.132 µM, OaAEP4 0.185 µM, OaAEP5 0.132 µM. The concentration of OaAEP1b was significantly higher as lower concentrations gave poor levels of conversion to cyclic product in the time frames tested. The assays were conducted in activity buffer (50 mM sodium acetate buffer, pH 5.0, 0.5 mM NaCl, 1 mM EDTA, 0.5 mM TCEP) at room temperature. The bar charts show mean values where n = 3 ± SEM. Reactions were stopped by heating at 70 °C for 5 min.