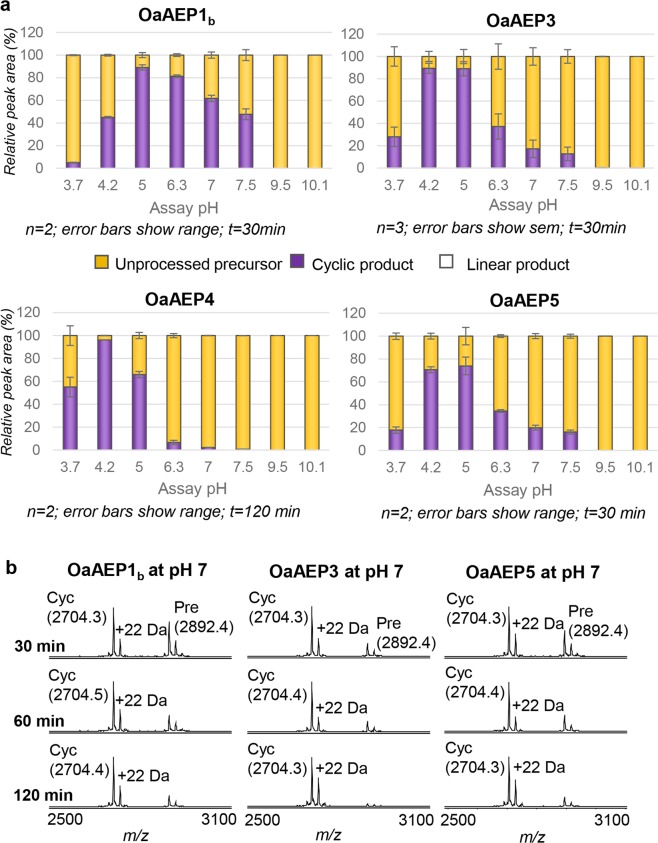
Figure 3.
Impact of pH on activity of recombinant AEP ligases. (a) Recombinant AEPs were incubated with the R1 peptide (GL–NGL variant; 280 µM) at a range of pH values and the products assessed by MALDI-MS. The relative peak area attributable to the cyclic product, linear product or unprocessed precursor was expressed as a % of the total peak area. Enzyme concentrations: OaAEP1b 0.528 µM, OaAEP3 0.132 µM, OaAEP4 0.033 µM, OaAEP5 0.132 µM. OaAEP1b was used at a higher concentration because it was not as active as the other enzymes under the conditions tested. The lower concentration of OaAEP4 reflects the low stock concentration of this enzyme. Reactions were stopped by heating at 70 °C for 5 min. The assay was conducted at room temperature using the same composition as the standard activity buffer (with 50 mM NaCl) but the buffering system was as appropriate for each pH and is detailed in the Materials and Methods. (b) Recombinant AEPs were incubated with the R1 peptide (KL–NGL variant; 280 µM) at pH 7 for 30, 60 or 120 min and the products assessed by MALDI-MS. The assays were performed in activity buffer (50 mM phosphate buffer, pH 7.0, 50 mM NaCl, 1 mM EDTA, 0.5 mM TCEP). The proportion of cyclic product was determined relative to all peaks attributed to the processed or unprocessed target peptide. OaAEP1b and OaAEP3 were at 0.528 µM; OaAEP5 was at 0.265 µM. Reactions were stopped by heating at 70 °C for 5 min. The observed monoisotopic masses are listed (Da; [M + H]+). The expected monoisotopic mass of cyclic product (cyc) is 3703.5 Da. The expected average mass of precursor (pre) is 2891.6 Da. +22 Da peaks present in precursor and product spectra are likely to represent a sodium adduct. A single representative experiment of two technical replicates is shown.