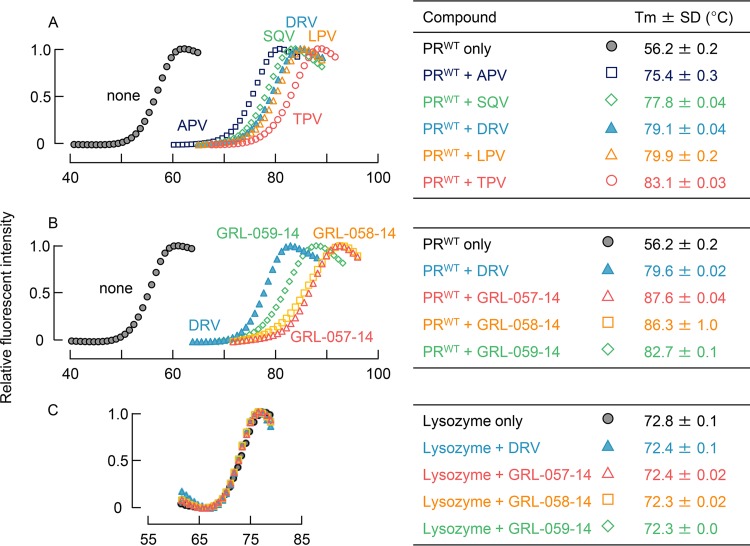
FIG 2.
Thermal stability of wild-type HIV-1 protease (PRWT) and lysozyme in the presence and absence of each compound using differential scanning fluorimetry (DSF). The relative fluorescence intensity was plotted at each temperature. Melting temperature (Tm) values were calculated from the top of the peak in the derivative plot. (A) Melting curves of PRWT in the presence and absence of selected FDA-approved PIs. Thermal stability curves with all the compounds shifted to higher temperatures than in the absence of each compound with Tm values ranging from 75.42 (amprenavir [APV]) to 83.08°C (tipranavir [TPV]), with a ΔTm value of 19.24°C to 26.90°C. (B) Melting curves of PRWT in the presence and absence of GRL-057-14, GRL-058-14, and GRL-059-14. The curve of DRV is shown as a reference. All three compounds significantly shifted the thermal stability curves to higher temperatures than in the absence of each compound. Their Tm values were much higher than those of all the FDA-approved PIs tested (A). (C) Melting curves of lysozyme in the presence and absence of GRL-057-14, GRL-058-14, GRL-059-14, and DRV. None of the PIs examined affected the thermal stability curves, indicating that all the PIs examined were highly specific to PRWT and had no significant interactions with lysozyme.