Figure 1.
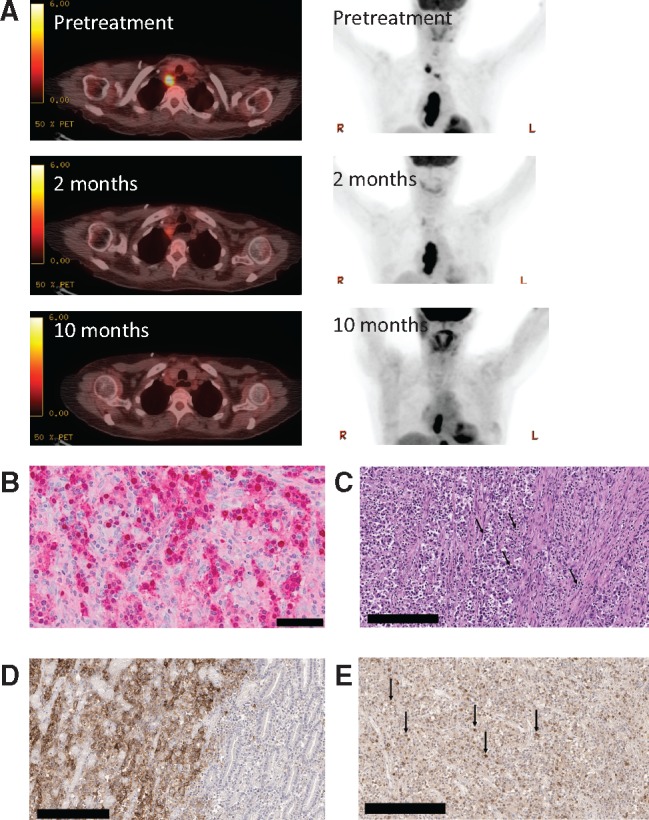
Histologic, radiologic, and genomic characteristics of a patient with Epstein-Barr virus (EBV)–positive gastric cancer responding to the anti–programmed death–ligand 1 (PD-L1) antibody therapy avelumab. A) Representative positron emission tomography–computerized tomography images taken prior to treatment with anti-PD-L1 antibody and two months and 10 months after initiation of therapy. B) Staining of primary tumor for EBV-encoded RNA (EBER) is shown in red. Normal gastric mucosa on the slide serves as an internal negative control (not shown). Scale bar = 50 μm. C) High-power image of the original gastric biopsy shows intense infiltrate of lymphocytes within the tumor (black arrows in the center) and associated stroma (black arrow to the right; hematoxylin and eosin ×400). Scale bar = 200 μm. D) Immunostaining of the gastric biopsy sample using the Ventana SP142 for PD-L1 antibody. Gastric adenocarcinoma with 100% staining of malignant cell in a membranous pattern (ie, only the peripheral cytoplasmic membrane stains for the marker and the nucleus and cytoplasm are unstained) for anti-PD-L1 (left portion of image). Bordering benign gastric mucosa shows complete absence of anti PD-L1 staining (right portion of image). The negative control omitting the anti-PD-L1 antibody showed no evidence of staining. Scale bar = 200 μm. E) Of the numerous tumor-infiltrating lymphocytes identified, 50% stain strongly positive for PD-1 expression (black arrows) using the OriGene PD-1 UltraMAB antibody. Scale bar = 200 μm.
