Figure 2.
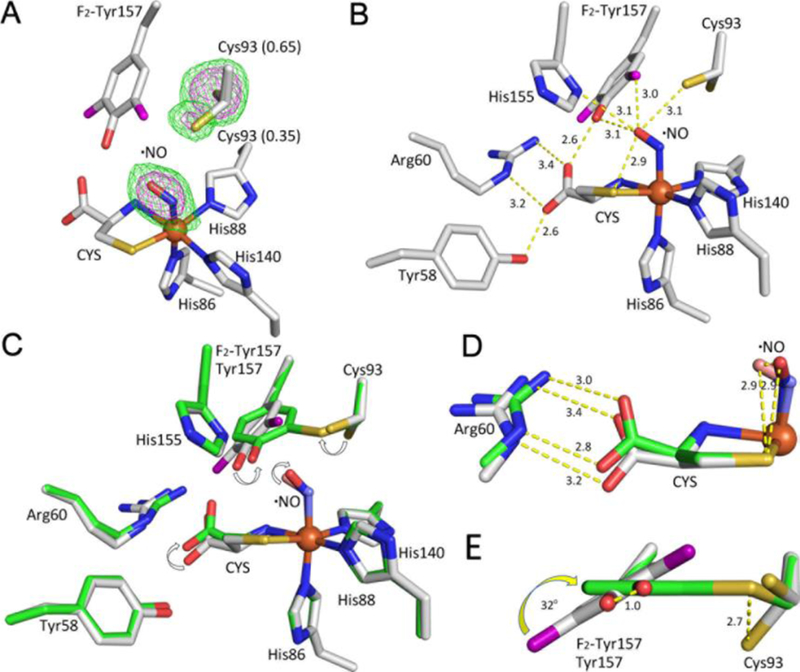
A ternary complex of the 100% uncrosslinked F2-Tyr157 hCDO bound with L-cysteine and •NO. The L-cysteine substrate is labeled as CYS. The fluorine atoms are shown in purple color. (A) The omit Fo−Fc electron densities of the •NO ligand and Cys93 (which has two conformations) contoured at 3 σ (green) and 6 σ (purple), respectively. (B) The details of the key interactions. The distances are in angstrom. (C) Alignment of the F2-Tyr157 CDO (grey) and the matured WT CDO (green). (D) Both the CYS and •NO rotate during the cofactor formation. (E) Tyr157 rotates during the cofactor biogenesis.
