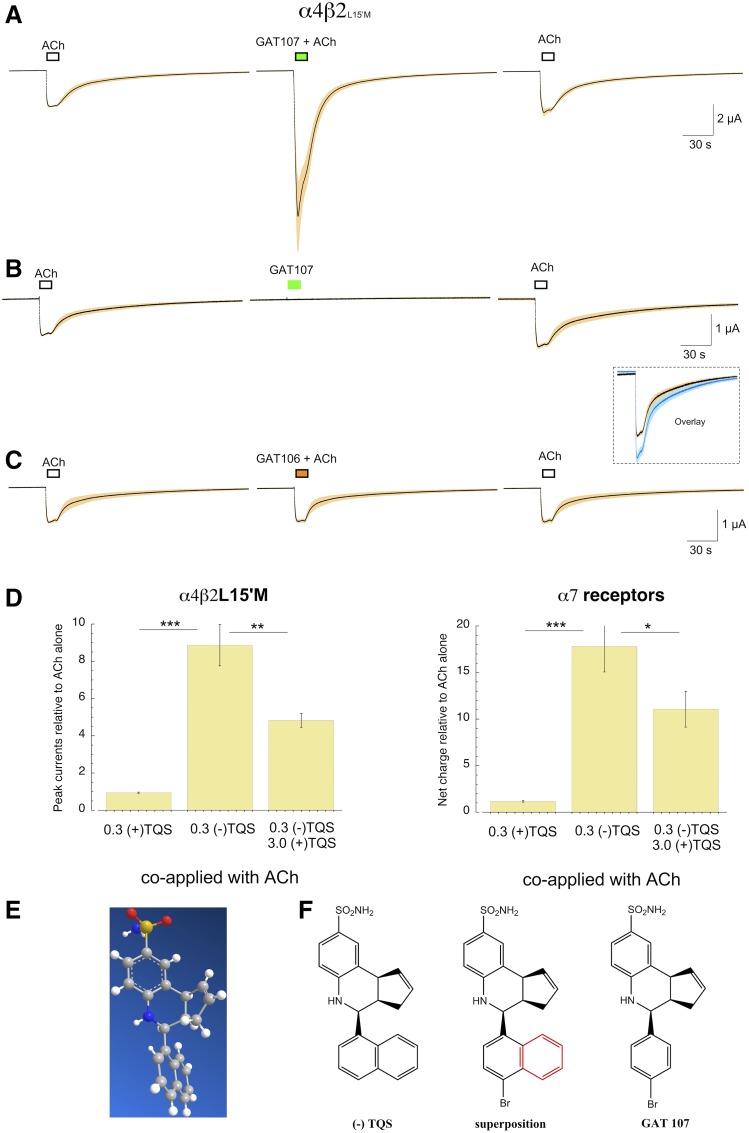
Fig. 5.
Responses of α4β2L15′M receptors to the ago-PAM GAT107 and its inactive isomer. (A) The coapplication 10 µM GAT107 with 30 µM ACh produced significant potentiation (P < 0.01 n = 8). (B) The application of 10 µM GAT107 alone did not produce allosteric activation of α4β2* receptors. However, when ACh was applied following the application of GAT107 alone, there was significant residual potentiation (P < 0.01 n = 7), as shown in the inset. (C) GAT106, the 4BP-TQS isomer that is inactive on α7 (Thakur et al., 2013), did not produce any potentiation of α4β2L15′M receptor ACh-evoked responses. (D) Activity of TQS isomers. Cells were injected with α4 and β2L15′M or wild-type α7. After two control applications of ACh (30 µM for α4β2L15′M or 60 µM for α7), ACh was coapplied with 300 nM of either the (+) or (−) isomer of TQS. Responses were normalized to the average of the ACh controls for each cell. (+)-TQS produced no significant potentiation compared with ACh alone, while (−)-TQS significantly (P < 0.001) potentiated responses of both receptor types. The addition of 3 µM (+)-TQS decreased responses compared with responses obtained with 300 nM (−)-TQS alone (P < 0.01, n = 7 for α4β2L15′M; P < 0.05, n = 8 for α7). α4β2L15′M responses were measured as peak currents; α7 responses were measured as net charge. (E) The X-ray crystal structure determined for (−)-TQS (see Materials and Methods). (F) The structure is for (−)-TQS displaying the absolute stereochemistry, followed by an overlay structure for TQS and GAT107 (the red color highlights the fused benzene ring found in TQS, but not in GAT107), and on the far right the structure is for GAT107 showing absolute stereochemistry.