Figure 4.
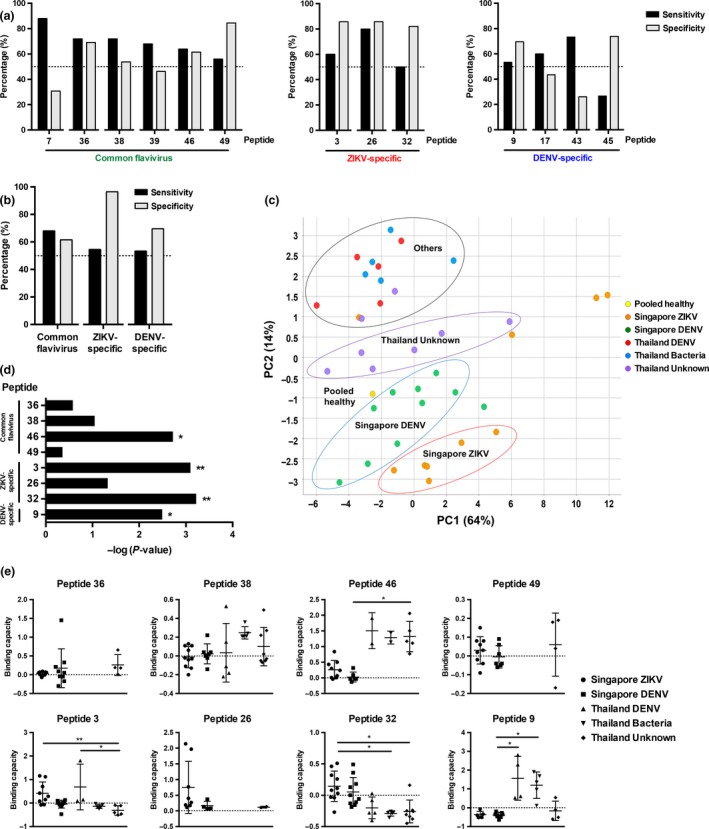
Preliminary diagnostic validation of identified linear B‐cell epitopes with patient cohorts. Convalescent plasma samples of ZIKV (n = 10) and serum samples of DENV (n = 10) patients from Singapore, and DENV (n = 5), bacteria (n = 5) and unknown (n = 8) patients from Thailand were tested in a peptide‐based ELISA in duplicates at 1:2000 dilution. Pooled healthy plasma was used as a negative control. (a) Sensitivity and specificity were determined for individual peptides. (b) Sensitivity and specificity of peptide mix of selected epitopes were determined. (c) Principal component analysis (PCA) of pooled healthy control, and patients’ anti‐IgG peptide response (OD values) were plotted in a graph with the percentage of variance indicated. (d, e) The peptide binding capacity of patients positively binding to peptides was calculated and statistically analysed by using the Kruskal–Wallis tests with the Bonferroni correction for multiple testing. Post hoc tests were done using Dunn's multiple comparison tests to determine (d) peptides with discriminating power and (e) the peptide binding capacity distribution of patients. Data are presented as mean ± SD. (*P < 0.05, **P < 0.01).
