This secondary analysis of the phase 1 CA209-003 clinical trial assesses the 5-year survival and other related factors among patients with advanced melanoma, renal cell carcinoma, or non–small cell lung cancer receiving nivolumab.
Key Points
Question
What is the 5-year survival, and what factors are associated with 5-year survival among patients with advanced melanoma, renal cell carcinoma, or non–small cell lung cancer receiving nivolumab?
Findings
In this secondary analysis of 270 patients from the CA209-003 clinical trial with advanced melanoma, renal cell carcinoma, or non–small cell lung cancer, 5-year survival was negatively associated with presence of bone or liver metastases and positively associated with Eastern Cooperative Oncology Group performance status of 0, objective response, degree of tumor burden reduction, and adverse event occurrence.
Meaning
Nivolumab treatment may be associated with durable survival among some heavily pretreated patients with advanced melanoma, renal cell carcinoma, or non–small cell lung cancer; characterizing factors associated with long-term survival may guide future anti–programmed cell death 1–based clinical trial design.
Abstract
Importance
Nivolumab, a monoclonal antibody that inhibits programmed cell death 1, is approved by the US Food and Drug Administration for treating advanced melanoma, renal cell carcinoma (RCC), non–small cell lung cancer (NSCLC), and other malignancies. Data on long-term survival among patients receiving nivolumab are limited.
Objectives
To analyze long-term overall survival (OS) among patients receiving nivolumab and identify clinical and laboratory measures associated with tumor regression and OS.
Design, Setting, and Participants
This was a secondary analysis of the phase 1 CA209-003 trial (with expansion cohorts), which was conducted at 13 US medical centers and included 270 patients with advanced melanoma, RCC, or NSCLC who received nivolumab and were enrolled between October 30, 2008, and December 28, 2011. The analyses were either specified in the original protocol or included in subsequent protocol amendments that were implemented between 2008 and 2012. Statistical analysis was performed from October 30, 2008, to November 11, 2016.
Intervention
In the CA209-003 trial, patients received nivolumab (0.1-10.0 mg/kg) every 2 weeks in 8-week cycles for up to 96 weeks, unless they developed progressive disease, achieved a complete response, experienced unacceptable toxic effects, or withdrew consent.
Main Outcomes and Measures
Safety and activity of nivolumab; OS was a post hoc end point with a minimum follow-up of 58.3 months.
Results
Of 270 patients included in this analysis, 107 (39.6%) had melanoma (72 [67.3%] male; median age, 61 [range, 29-85] years), 34 (12.6%) had RCC (26 [76.5%] male; median age, 58 [range, 35-74] years), and 129 (47.8%) had NSCLC (79 [61.2%] male; median age, 65 [range, 38-85] years). Overall survival curves showed estimated 5-year rates of 34.2% among patients with melanoma, 27.7% among patients with RCC, and 15.6% among patients with NSCLC. In a multivariable analysis, the presence of liver (odds ratio [OR], 0.31; 95% CI, 0.12-0.83; P = .02) or bone metastases (OR, 0.31; 95% CI, 0.10-0.93; P = .04) was independently associated with reduced likelihood of survival at 5 years, whereas an Eastern Cooperative Oncology Group performance status of 0 (OR, 2.74; 95% CI, 1.43-5.27; P = .003) was independently associated with an increased likelihood of 5-year survival. Overall survival was significantly longer among patients with treatment-related AEs of any grade (median, 19.8 months; 95% CI, 13.8-26.9 months) or grade 3 or more (median, 20.3 months; 95% CI, 12.5-44.9 months) compared with those without treatment-related AEs (median, 5.8 months; 95% CI, 4.6-7.8 months) (P < .001 for both comparisons based on hazard ratios).
Conclusions and Relevance
Nivolumab treatment was associated with long-term survival in a subset of heavily pretreated patients with advanced melanoma, RCC, or NSCLC. Characterizing factors associated with long-term survival may inform treatment approaches and strategies for future clinical trial development.
Trial Registration
ClinicalTrials.gov identifier: NCT00730639
Introduction
Clinical trials of antibodies targeting programmed cell death 1 (PD-1), an inhibitory receptor expressed on activated lymphocytes, or its major ligand, PD-L1, show activity across a broad range of advanced cancers.1 Antibodies blocking PD-1 or PD-L1 are currently approved by the US Food and Drug Administration for treating multiple individual cancer types in the advanced or metastatic disease setting and for the broad, genetically defined category of microsatellite instability-high tumors.2 They are also approved in the adjuvant setting for melanoma and are under investigation in adjuvant and/or neoadjuvant protocols in various tumor types. Anti–PD-L1 treatment is generally well tolerated, with grade 3 to 4 treatment-related adverse events (AEs) observed in approximately 10% to 20% of patients.3,4,5
The first multidose trial (CA209-003)6 of a PD-1/PD-L1 antagonist, the anti–PD-1 nivolumab, was initiated on October 30, 2008. Combined results from the dose-escalation and dose-expansion cohorts in 5 types of advanced cancers—melanoma, renal cell carcinoma (RCC), non–small cell lung cancer (NSCLC), colorectal cancer, and prostate cancer—were first reported in 2012.7 No objective tumor regressions were observed among patients with colorectal or prostate cancer in this trial.7 Individual analyses of the completed melanoma, RCC, and NSCLC cohorts were subsequently published,8,9,10 reporting objective response, progression-free survival, and preliminary overall survival (OS) results with 2 to 3 years of follow-up. More recently, 5-year follow-up data from the NSCLC cohort were reported.11 Here, we assessed long-term survival outcomes among 270 patients with advanced melanoma, RCC, and NSCLC in the CA209-003 trial. In addition, we sought to identify baseline and on-treatment factors associated with long-term OS by examining clinical characteristics, AE occurrence, depth of tumor regression, and circulating lymphocyte counts in these patients.
Methods
Patients
This was a secondary analysis of the CA209-003 trial.6 Analyses were either specified in the original protocol (Supplement 1) or included in subsequent protocol amendments that were implemented between 2008 and 2012. Eligibility criteria for this study have been described previously.7 In brief, patients 18 years or older with documented evidence of advanced melanoma, RCC, NSCLC, castration-resistant prostate cancer, or colorectal cancer were enrolled between October 30, 2008, and December 28, 2011. Eligible patients had received 1 to 5 previous systemic therapies for advanced or recurrent cancer, had measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0,12 and an Eastern Cooperative Oncology Group (ECOG)13 performance status of 0 to 2. Patients with untreated brain metastases were ineligible. Patients with autoimmune diseases, with conditions requiring immunosuppressive medications, or previously treated with T-cell modulating antibodies (eg, anti–PD-1/PD-L1 and anti–CTLA-4) were excluded. The protocol was approved by local institutional review boards at participating sites, and the study was conducted in accordance with international standards of good clinical practice. Patients or their legal representatives provided written informed consent before enrollment. Additional data were collected after the protocol-defined study period as allowed by participating institutions. Statistical analysis was performed from October 30, 2008, to November 11, 2016.
Study Design and Treatment
The CA209-003 trial was a multicenter, phase 1, dose-escalation, cohort-expansion trial conducted at 13 medical centers in the United States. Based on preliminary evidence of activity, expansion cohorts were enrolled for melanoma, RCC, and NSCLC (squamous and non-squamous subtypes).7 Patients received nivolumab intravenously every 2 weeks in 8-week cycles for up to 96 weeks (12 cycles) or until unacceptable toxic effects occurred, confirmed complete response, confirmed progressive disease, or consent withdrawal. Doses of nivolumab administered to patients with melanoma were 0.1, 0.3, 1.0, 3.0, or 10.0 mg/kg; patients with RCC, 1.0 or 10.0 mg/kg; and patients with NSCLC, 1.0, 3.0, or 10.0 mg/kg. Patients with stable disease or tumor regression while receiving nivolumab therapy and who had disease progression within 1 year of discontinuation could resume therapy for up to 1 additional year.
Assessments and Analyses
In the CA209-003 trial, radiographic tumor assessments were performed at screening, after each treatment cycle, and every 8 weeks after completing therapy for up to 1 year or until relapse or initiation of a new therapy. Response was assessed using RECIST, version 1.0.12 Patients were followed up for assessment of progression-free survival for up to 3 years and, for OS, every 3 months for an indefinite period either in-person or by phone. For patients who survived for 5 or more years after treatment initiation, information about tumor response status after the protocol-specified follow-up period and about subsequent treatments received was provided by the investigators when available.
For analyses of patients treated or not treated beyond disease progression, treated beyond progression was defined as treatment with nivolumab for 4 or more weeks after progression, and not treated beyond progression was defined as either no nivolumab therapy or less than 4 weeks of nivolumab therapy after progression. Subsequent changes in tumor burden were measured with respect to maximum tumor measurements at progression.
Safety data were collected for all treated patients every 2 weeks during therapy and for up to 70 days after the last dose of nivolumab. The Medical Dictionary for Regulatory Activities (MedDRA, version 15.1),14 was used to code AEs, and the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0,15 was used to grade the severity of AEs. A sponsor-derived prespecified list of MedDRA terms in 7 categories was used to identify select AEs, defined as AEs of potential immune-mediated causality that require frequent monitoring or intervention with immunosuppression or replacement therapy.8
Analysis of baseline immune cell counts was conducted on whole blood samples collected immediately before treatment. Absolute lymphocyte counts (ALCs) were determined from automated complete blood cell counts conducted at study site clinical laboratories. To enumerate regulatory T cells, whole blood samples were treated to lyse erythrocytes and were incubated with phenotype-associated antibodies. Standard flow cytometric detection and analysis were used to determine absolute regulatory T-cell counts, with regulatory T cells defined as CD3+CD4+CD25bright cells.
Statistical Analysis
Time-to-event measures (duration of response and OS) from time of the first nivolumab dose were estimated using Kaplan-Meier methods. For objective response rate (ORR), exact binomial 95% CIs were determined using Clopper-Pearson methods. Unstratified hazard ratios (HRs) and 95% CIs based on a Cox proportional hazards regression model were provided for univariate analyses; odds ratios (ORs) and 95% CIs based on logistic regression were provided for multivariable analyses. Covariates included age, sex, ECOG performance status, baseline metastatic sites, and number of previous therapies. Similar modeling methods were used for statistical comparisons of efficacy outcomes based on treatment-related AE incidence and for comparisons of baseline immune cell counts between patients who responded (complete response or partial response) and those who do not respond to treatment and between patients alive or dead at 5 years. Comparisons of baseline sum of target lesion diameters between patients alive or dead at 5 years were based on t tests. Two-sided significance levels (nominal threshold of 0.05) were reported, and SAS software, version 8.2 (SAS Institute Inc) was used for statistical analysis. Reported analyses, including OS, were exploratory in nature; therefore, P values should be interpreted with caution.
Results
Patient Characteristics
Of 270 patients with advanced cancers who received nivolumab and were evaluated, 107 (39.6%) had melanoma (72 [67.3%] male; median age, 61 years [range, 29-85 years]), 34 (12.6%) had RCC (26 [76.5%] male; median age, 58 years [range, 35-74 years]), and 129 (47.8%) had NSCLC (79 [61.2%] male; median age, 65 years [range, 38-85 years]). Baseline patient characteristics by disease type have been detailed7,8,9,10,11 and are summarized in eTable 1 in Supplement 2. Overall, most patients were heavily pretreated, with 109 (40.4%) receiving 3 or more previous regimens. Median number of nivolumab doses received on study was 11 doses (range, 1-50 doses) for patients with melanoma, 16 doses (2-48 doses) for patients with RCC, and 6 doses (1-51 doses) for patients with NSCLC.
Objective Response
The ORR (complete response and partial response) to nivolumab treatment was 31.8% for patients with melanoma (34 of 107 patients), 29.4% (10 of 34) for RCC, and 17.1% (22 of 129) for NSCLC (Table). Responses were durable in all 3 tumor types, with median durations of 22.9 months (95% CI, 16.7-31.8 months) for melanoma, 12.9 months (95% CI, 8.4 months to not estimable) for RCC, and 19.1 months (8.7 months to not estimable) for NSCLC (Figure 1A). Additional patients experienced stable disease lasting 6 months or more. Disease control rate (complete response, partial response, and stable disease for 6 months or more) was 53.3% (57 of 107 patients) for melanoma, 70.6% (24 of 34) for RCC, and 41.9% (54 of 129) for NSCLC (Table).
Table. Response to Treatment.
| Response Assessmentsa | Patients, No. (%) | ||
|---|---|---|---|
| Melanoma (n = 107) | RCC (n = 34) | NSCLC (n = 129) | |
| Objective responseb | 34 (31.8) | 10 (29.4) | 22 (17.1) |
| 95% CI for response rate, % | 23.1-41.5 | 15.1-47.5 | 11.0-24.7 |
| Best overall response | |||
| Complete response | 2 (1.9) | 1 (2.9) | 0 |
| Partial response | 32 (29.9) | 9 (26.5) | 22 (17.1) |
| Stable disease ≥6 mo | 23 (21.5) | 14 (41.2) | 32 (24.8) |
| Progressive disease | 41 (38.3) | 8 (23.5) | 59 (45.7) |
| Unable to determine | 9 (8.4) | 2 (5.9) | 16 (12.4) |
| Disease control ratec | 57 (53.3) | 24 (70.6) | 54 (41.9) |
Abbreviations: NSCLC, non–small cell lung cancer; RCC, renal cell carcinoma; RECIST, Response Evaluation Criteria in Solid Tumors.
Response assessments are defined by modified RECIST, version 1.0.12
Complete response and partial response.
Complete response, partial response, and stable disease for 6 months or more.
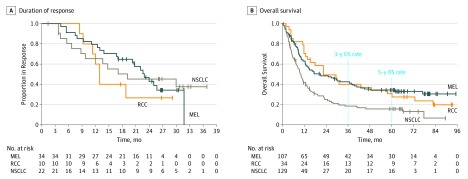
Figure 1. Durability of Tumor Regression and Overall Survival (OS) Among Patients With Advanced Melanoma (MEL), Renal Cell Carcinoma (RCC), or Non-Small Cell Lung Cancer (NSCLC) Receiving Nivolumab.
A, Kaplan-Meier estimates of duration of response among 66 patients achieving a complete or partial tumor response with nivolumab therapy. Estimates of response duration are truncated at 3 years based on the protocol-defined maximum treatment interval of 96 weeks and follow-up period of 46 weeks. B, Kaplan-Meier estimates of OS among 270 patients. Hash marks indicate censored events, defined for OS as the time to the last known alive date before the date of data analysis for patients without a recorded death.
Overall Survival
Minimum follow-up for OS for melanoma was 58.3 months, for RCC was 63.9 months, and for NSCLC was 58.3 months. Median OS was 20.3 months (95% CI, 12.5-37.9 months) for patients with melanoma, 22.4 months (95% CI, 12.5-48.6 months) for patients with RCC, and 9.9 months (95% CI, 7.8-12.4 months) for patients with NSCLC (Figure 1B). Overall survival curves showed an inflection toward flattening around 3 years after treatment initiation, with estimated 3-year OS rates of 42.3% (95% CI, 32.7%-51.6%) for melanoma, 40.1% (95% CI, 23.6%-56.0%) for RCC, and 18.4% (95% CI, 11.9%-26.0%) for NSCLC and 5-year OS rates of 34.2% (95% CI, 25.2%-43.4%) for melanoma, 27.7% (95% CI, 13.9%-43.5%) for RCC, and 15.6% (95% CI, 9.6%-22.9%) for NSCLC. More detailed analyses of 5-year OS rates for patients with squamous subtype (16%) or nonsquamous subtype (15%) of NSCLC were previously reported.11 Overall survival curves according to nivolumab dose level are shown in eFigure 1 in Supplement 2.
Factors Associated With Long-term Survival
Baseline Demographic and Clinical Characteristics
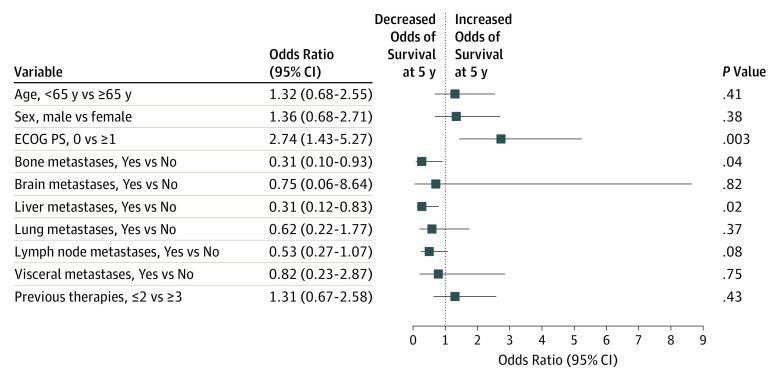
Univariate and multivariable analyses were performed using data from all 270 patients to assess potential associations between baseline demographic and clinical characteristics and 5-year OS. In univariate analyses including all patients, an ECOG performance status of 0 (vs ≥1) was associated with increased 5-year survival (HR, 0.53; 95% CI, 0.40-0.71). Bone (HR, 1.58; 95% CI, 1.13-2.20), liver (HR, 1.78; 95% CI, 1.31-2.41), lung (HR, 1.42; 95% CI, 1.00-2.01), or visceral metastases (HR, 1.78; 95% CI, 1.13-2.79) were associated with decreased survival (eTable 2 in Supplement 2). In a multivariable analysis including all patients, baseline presence of liver (OR, 0.31; 95% CI, 0.12-0.83; P = .02) or bone metastases (OR, 0.31; 95% CI, 0.10-0.93; P = .04) was independently associated with a decreased likelihood of survival at 5 years (Figure 2). Furthermore, greater baseline tumor burden, based on the sum of target lesion diameters, was associated with lower 5-year survival; median sum of target lesion diameters was 88.0 mm (interquartile range, 52.0-116.0 mm) among patients alive at 5 years vs 109.0 mm (interquartile range, 65.0-165.0 mm) among patients who were dead at 5 years (P < .02) (eTable 3 in Supplement 2). Conversely, ECOG performance status (0 vs ≥1) was independently associated with an increased likelihood of 5-year survival (OR, 2.74; 95% CI, 1.43-5.27; P = .003). Across multiple univariate and multivariable analyses conducted, including either individual tumor-type populations or all 270 patients, patient age (<65 vs ≥65 years or <75 vs ≥75 years) and sex, presence or absence of brain or lymph node metastases, and the number of previous therapies (>2 vs ≤2) were not statistically associated with long-term survival among patients treated with nivolumab. However, patients with untreated brain metastases were excluded from this trial.
Figure 2. Associations of Baseline Demographic and Clinical Characteristics With Overall Survival at 5 Years.
Multivariable logistic regression analysis based on 55 alive patients and 215 dead patients at 5 years. For baseline metastases, the comparison represents patients with the selected metastatic site (either a solitary site or in the presence of other metastatic sites) vs patients without the selected metastatic site. ECOG PS indicates Eastern Cooperative Oncology Group performance status.
Tumor Response
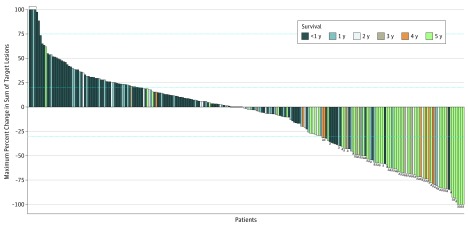
Among 245 patients with available radiographic data, the degree of total reduction in tumor burden measured in target lesions appeared to be directly associated with long-term survival (Figure 3). In support of this observation, there was a significant association between ORR and 5-year survival; an objective response was achieved by 41 of 55 patients (74.5%) alive at 5 years vs 25 of 215 patients (11.6%) who were dead at 5 years (OR, 22.3; 95% CI, 10.7-46.5; P < .001) (eTable 4 and eTable 5 in Supplement 2). Swimmer plots depicting individual responders are provided in eFigure 2 in Supplement 2.
Figure 3. Association Between Tumor Target Lesion Reduction and Overall Survival.
The maximum percent change is depicted for 245 patients who had measurable target lesions at baseline and at least 1 on-treatment tumor assessment. Horizontal dashed lines indicate a 20% increase and 30% reduction in target lesion measurements. Bracket at 100% denotes tumor growth truncated at 100%.
aComplete or partial response.
Adverse Events
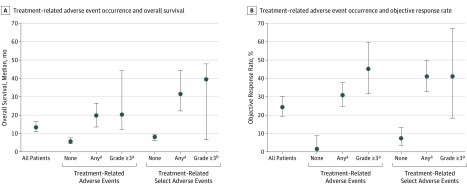
Long-term safety outcomes among patients treated with nivolumab in the CA209-003 trial have been previously reported.7,8,9,10 In this analysis, among 270 patients with advanced melanoma, RCC, or NSCLC, treatment-related AEs of any grade were experienced by 210 patients (77.8%), grade 3 or more treatment-related AEs by 53 patients (19.6%), treatment-related select AEs of any grade by 136 patients (50.4%), and grade 3 or more treatment-related select AEs by 17 patients (6.3%) (eTable 6 in Supplement 2). There were 3 treatment-related deaths, all among patients with NSCLC.10 Overall survival was significantly longer among patients experiencing treatment-related AEs of any grade (median, 19.8 months; 95% CI, 13.8-26.9 months) or grade 3 or more (median, 20.3 months; 95% CI, 12.5-44.9 months) compared with patients not experiencing any treatment-related AEs (median, 5.8 months; 95% CI, 4.6-7.8 months; P < .001 for both comparisons based on HRs) (Figure 4A). In addition, OS was significantly longer among patients experiencing treatment-related select AEs of any grade (median, 31.6 months; 95% CI, 22.7-44.9 months; P < .001 based on HRs) or grade 3 or more (median, 39.6 months; 95% CI, 6.7-48.6 months; P < .05 based on HRs) vs those who did not experience any treatment-related select AEs (median, 8.2 months; 95% CI, 6.2-9.5 months) (Figure 4A). A similar association was observed between treatment-related AEs or treatment-related select AEs and ORR (Figure 4B). Results for the individual tumor types are shown in eFigure 3 in Supplement 2.
Figure 4. Association Between Incidence of Treatment-Related Adverse Events and Clinical Outcomes in 270 Patients With Melanoma, RCC, or NSCLC Receiving Nivolumab.
Analysis includes all treatment-related adverse events occurring between administration of the first dose of nivolumab and 30 days after the last dose. Incidence of treatment-related adverse events was not controlled for drug exposure. Treatment-related select adverse events are defined as those with a potential immune-mediated causality.8 P values were determined using a Cox proportional hazards regression model and are based on the hazard ratio for survival (none vs any or grade ≥3) subgroups. Error bars represent 95% CIs.
aP < .001.
bP < .05 vs none in the respective treatment-related adverse event category.
Baseline ALC and Regulatory T Cells
Patients with objective response (complete response and partial response) had a significantly higher mean baseline ALC vs patients without response (1480 vs 1300 cells/μL, P = .04). Furthermore, the proportion of patients with baseline ALC below the lower limit of normal (850 cells/μL) was less in patients who responded vs those who did not respond (16.9% vs 25.2%). In contrast, there was no statistically significant difference between those with objective response and those without response in association with mean baseline numbers of circulating regulatory T cells (42.4 vs 34.2 cells/μL, P = .18) (eFigure 4 in Supplement 2). Patients alive at 5 years had a significantly higher mean baseline ALC compared with patients who were dead at 5 years (1546 vs 1290 cells/μL; P = .01) and a significantly higher mean baseline number of circulating regulatory T cells (49.5 vs 32.6 cells/μL; P = .02) (eFigure 5 in Supplement 2).
Treatment Beyond Progression
Forty-three patients experiencing tumor progression during treatment cycles 1 or 2 continued to receive nivolumab on-study. Among them, 23 had at least 1 tumor assessment after progression, and only 4 of these patients (2 with melanoma, 1 with RCC, 1 with NSCLC) showed a maximum tumor burden reduction of 30% or more compared with tumor target lesion measurements at progression; another 4 patients showed a tumor burden reduction of less than 30%, and 13 patients showed tumor burden increases of less than 20% (eFigure 6 in Supplement 2). The CA209-003 study was, to our knowledge, the first trial of any anti–PD-1/PD-L1 drug to use treatment beyond progression. Our findings that most patients treated in this manner did not achieve an objective response are similar to those presented in a recent pooled analysis of 2624 patients with advanced melanoma treated with anti–PD-1, which found a 14% ORR in those treated beyond progression.16
Discussion
Nivolumab was the first PD-1 pathway blocking drug to enter clinical testing in a first–in-human trial commencing in 2006.17 Since then, application of anti–PD-L1 drugs has expanded to multiple tumor indications, firmly substantiating the role of PD-1 as a clinically validated target for cancer therapy.18 We report, to our knowledge, the longest combined clinical follow-up for patients with multiple cancer types receiving any anti–PD-L1 drug. While short-term clinical outcomes from this trial, such as ORR and progression-free survival, have been previously reported,7,8,9,10 the current study extends these findings to reveal a subset of patients remaining alive at 5 years. The results of this study suggest that survival benefits reported in the more limited follow-up of recent nivolumab randomized clinical trials may persist for prolonged periods in some patients, extending to at least 5 years. Of note, all patients enrolled in this study had received at least 1 previous systemic cancer therapy and 71.9% of patients had received 2 or more systemic cancer therapies. The estimated 5-year survival rates of 34.2% for melanoma, 27.7% for RCC, and 15.6% for NSCLC exceed survival rates expected from conventional second-line or third-line therapies available for patients at the time that this trial was conducted. For instance, in 2008, the expected 3-year survival rate for patients with stage IV melanoma was approximately 5%,19,20 and the anticipated 5-year survival rate for patients with stage IV NSCLC was approximately 6%.21 Although information about subsequent therapy was not available for all patients alive at 5 years, in a previous detailed assessment of the 16 patients with NSCLC who survived for 5 years, 12 (75.0%) were reported to have a partial response and had not received subsequent therapy as of a November 2016 database lock.11
The current report provides preliminary evidence for early surrogate markers of long-term clinical benefit. Such markers may be valuable on a per-patient basis and in designing the conduct and assessment of future trials of anti–PD-1 drugs. We examined baseline and on-treatment measures for potential associations with long-term OS. Among pretreatment clinical variables in the multivariable analysis, an ECOG performance status of 1 or more, bone metastases, and liver metastases were significantly associated with decreased 5-year OS. Previous reports have associated liver metastases with reduced ORR and progression-free survival among patients with advanced melanoma receiving anti–PD-1 therapy.22,23 However, in contrast to other studies, we did not find that patient age or number of previous systemic therapies was associated with outcomes in this pretreated population.24 Among pretreatment laboratory tests, an association between tumor cell PD-L1 expression and objective response to nivolumab was previously reported in the CA209-003 trial.7 Subsequent validation of these findings supported US Food and Drug Administration approvals for PD-L1 immunohistochemistry tests in NSCLC and several other cancers eligible for anti–PD-(L)1 therapies.1 In the current analysis, a higher pretreatment ALC was significantly associated with objective response and 5-year survival among the 270 patients with advanced melanoma, RCC, or NSCLC receiving nivolumab. Increased on-treatment ALCs were initially shown to be associated with longer OS among patients with advanced melanoma receiving a different immune checkpoint inhibitor, ipilimumab (anti–CTLA-4).25 Although some studies have failed to show an association between ALC and anti–PD-1 clinical outcomes,26 more recent reports suggest that a low baseline ALC may be associated with reduced progression-free survival.27,28
Among on-treatment measurements, we found an association between objective tumor regression (complete response and partial response) and 5-year OS and between a greater percent reduction in total tumor burden and increased long-term survival. These findings favor the general assumption that objective tumor regression provides an early surrogate for long-term clinical benefit, in contrast to a recent meta-analysis of checkpoint inhibitor trials showing a poor association between ORR and 12-month OS.29 Of note, patients in our study who developed treatment-related AEs, regardless of whether the AEs were deemed to have an immune-mediated causality, had significantly higher ORRs and prolonged 5-year OS. These findings are reminiscent of some reports of anti–CTLA-4 therapy30 and align with other studies of anti–PD-1 therapies.31,32,33 Moreover, they highlight the possibility that anti–PD-1–mediated tumor regression and toxic effects are functionally associated. This may occur through antigen mimicry or identity or may simply reflect a concomitant disinhibition of antitumor immunity and autoimmunity. Furthermore, maintenance of ongoing tumor regressions reported in patients with early discontinuation of anti–PD-1 therapy because of AEs3 suggests that treatment periods shorter than 1 to 2 years may be as efficacious as longer regimens in some patients. Recent studies showing rapid antitumor effects of anti–PD-1 in the neoadjuvant setting support this possibility.34,35
Finally, milestone analysis of survival rates has been suggested as a relatively short-term predictor of long-term outcomes from anti–PD-1 therapy.36 Findings from the current study, showing a flattening of the OS curves for advanced melanoma, RCC, and NSCLC around 3 years after treatment initiation, support this concept. The contours of these curves for 3 different cancer types are remarkably similar to each other and to results from trials of anti–PD-1 therapy for individual tumor types.37,38 Furthermore, these curves suggest durable clinical benefit even after treatment discontinuation at 2 years, similar to a recent report of pembrolizumab in patients with advanced melanoma who experienced a complete response.39 The extension of follow-up periods to 5 years and beyond, as provided here and in other recent publications,38,40 suggests the long-term durability of the treatment effects of anti–PD-1.
Limitations
Although data from this study provide a unique insight into the long-term survival outcomes for patients treated with nivolumab, it is important to consider the limitations of the study including the post hoc nature of some of the analyses, and the fact that different doses of nivolumab were administered to different cohorts of patients in this dose-escalation trial. Furthermore, the study population of 270 patients includes a relatively small number of patients with RCC compared with melanoma or NSCLC. Although results from analyses of individual tumor types are provided, the main conclusions are drawn from combined analyses of all 270 patients and could be influenced by tumor type-specific phenomena.
Conclusions
Nivolumab treatment was associated with long-term survival in a subset of heavily pretreated patients with advanced melanoma, RCC, or NSCLC. Characterizing factors associated with long-term survival may inform treatment approaches for individual patients and strategies for future clinical trial development in immuno-oncology.
Trial Protocol
eTable 1. Baseline Patient Characteristics (N = 270)
eTable 2. Univariate Analysis of Associations Between Baseline Demographic or Clinical Characteristics and Overall Survival at 5 Years in 270 patients With Melanoma, RCC, or NSCLC Receiving Nivolumab
eTable 3. Association Between Baseline Sum of Target Lesion Diameters and 5-Year Survival in All Patients Receiving Nivolumab (N = 270)
eTable 4. Association Between Objective Response and 5-Year Survival in Patients With Melanoma, RCC, or NSCLC Receiving Nivolumab (N = 270)
eTable 5. Association Between Objective Response and 5-Year Survival in all Patients Receiving Nivolumab (N = 270)
eTable 6. Summary of Treatment-related Adverse Events in All Patients Receiving Nivolumab (N = 270)
eFigure 1. Overall Survival In Patients With Melanoma, RCC, or NSCLC Based on Nivolumab Dose Received
eFigure 2. Swimmer Plots for Responders With Advanced Melanoma, RCC, or NSCLC
eFigure 3. Association Between Incidence of Treatment-Related Adverse Events and Clinical Outcomes in Patients With Melanoma, RCC, or NSCLC Receiving Nivolumab
eFigure 4. Association Between Baseline Immune Cell Counts and Objective Response in Patients With Melanoma, RCC, or NSCLC Receiving Nivolumab
eFigure 5. Association Between Baseline Immune Cell Counts and 5-Year Survival in Patients With Melanoma, RCC, or NSCLC Receiving Nivolumab
eFigure 6. Maximum Change in Tumor Burden in 23 Patients With Melanoma, RCC, or NSCLC Who Were Treated With Nivolumab Beyond Tumor Progression
References
- 1.Topalian SL. Targeting immune checkpoints in cancer therapy. JAMA. 2017;318(17):1647-1648. doi: 10.1001/jama.2017.14155 [DOI] [PubMed] [Google Scholar]
- 2.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6(1):8. doi: 10.1186/s40425-018-0316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785-792. doi: 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
- 4.Luo W, Wang Z, Tian P, Li W. Safety and tolerability of PD-1/PD-L1 inhibitors in the treatment of non–small cell lung cancer: a meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol. 2018;144(10):1851-1859. doi: 10.1007/s00432-018-2707-4 [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Seo HK. Immune checkpoint inhibitors for urothelial carcinoma. Investig Clin Urol. 2018;59(5):285-296. doi: 10.4111/icu.2018.59.5.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ClinicalTrials.gov . A phase 1 study of nivolumab (BMS-936558) in subjects with advanced or recurrent malignancies (MDX1106-03). CA209-003; NCT00730639. http://clinicaltrials.gov/ct2/show/NCT00730639. Accessed June 20, 2019.
- 7.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020-1030. doi: 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33(18):2013-2020. doi: 10.1200/JCO.2014.58.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non–small-cell lung cancer. J Clin Oncol. 2015;33(18):2004-2012. doi: 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of nivolumab in previously treated advanced non–small-cell lung cancer: results from the CA209-003 study. J Clin Oncol. 2018;36(17):1675-1684. doi: 10.1200/JCO.2017.77.0412 [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205-216. doi: 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 13.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655. doi: 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 14.Cancer Therapy Evaluation Program (CTEP) Introductory Guide MedDRA Version 15.1. MSSO-DI-6003-15.1.0; September 2012. https://www.meddra.org/sites/default/files/guidance/file/intguide_15_1_English_0.pdf. Accessed on June 20, 2019.
- 15.Cancer Therapy Evaluation Program (CTEP) Common Terminology Criteria for Adverse Event v3.0 (CTCAE). Washington, DC: Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, Department of Healthand Human Services; August 9, 2006. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed June 20, 2019.
- 16.Beaver JA, Hazarika M, Mulkey F, et al. Patients with melanoma treated with an anti–PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19(2):229-239. doi: 10.1016/S1470-2045(17)30846-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167-3175. doi: 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450-461. doi: 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527-534. doi: 10.1200/JCO.2007.12.7837 [DOI] [PubMed] [Google Scholar]
- 20.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstraw P, Chansky K, Crowley J, et al. ; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions . The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51. doi: 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 22.Nosrati A, Tsai KK, Goldinger SM, et al. Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer. 2017;116(9):1141-1147. doi: 10.1038/bjc.2017.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti–PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417-424. doi: 10.1158/2326-6066.CIR-16-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kugel CH III, Douglass SM, Webster MR, et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res. 2018;24(21):5347-5356. doi: 10.1158/1078-0432.CCR-18-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767-1775. doi: 10.1002/cncr.24951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman CF, Postow MA. Emerging tissue and blood-based biomarkers that may predict response to immune checkpoint inhibition. Curr Oncol Rep. 2016;18(4):21. doi: 10.1007/s11912-016-0509-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017;8(69):114268-114280. doi: 10.18632/oncotarget.23217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho WJ, Yarchoan M, Hopkins A, Mehra R, Grossman S, Kang H. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J Immunother Cancer. 2018;6(1):84. doi: 10.1186/s40425-018-0395-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchie G, Gasper H, Man J, et al. Defining the most appropriate primary end point in phase 2 trials of immune checkpoint inhibitors for advanced solid cancers: a systematic review and meta-analysis. JAMA Oncol. 2018;4(4):522-528. doi: 10.1001/jamaoncol.2017.5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043-6053. doi: 10.1200/JCO.2005.06.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shafqat H, Gourdin T, Sion A. Immune-related adverse events are linked with improved progression-free survival in patients receiving anti–PD-1/PD-L1 therapy. Semin Oncol. 2018;45(3):156-163. doi: 10.1053/j.seminoncol.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 32.Lisberg A, Tucker DA, Goldman JW, et al. Treatment-related adverse events predict improved clinical outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer Immunol Res. 2018;6(3):288-294. doi: 10.1158/2326-6066.CIR-17-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogado J, Sánchez-Torres JM, Romero-Laorden N, et al. Immune-related adverse events predict the therapeutic efficacy of anti–PD-1 antibodies in cancer patients. Eur J Cancer. 2019;109:21-27. doi: 10.1016/j.ejca.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 34.Forde PM, Chaft JE, Pardoll DM. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;379(9):e14. doi: 10.1056/NEJMc1808251 [DOI] [PubMed] [Google Scholar]
- 35.Topalian SL, Bhatia S, Kudchadkar RR, et al. Nivolumab (nivo) as neoadjuvant therapy in patients with resectable Merkel cell carcinoma (MCC) in CheckMate 358. J Clin Oncol. 2018;36(15)(suppl):9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumenthal GM, Zhang L, Zhang H, et al. Milestone analyses of immune checkpoint inhibitors, targeted therapy, and conventional therapy in metastatic non–small cell lung cancer trials: a meta-analysis. JAMA Oncol. 2017;3(8):e171029. doi: 10.1001/jamaoncol.2017.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long GV, Schachter J, Ribas A, et al. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in KEYNOTE-006. J Clin Oncol. 2018;36(15)(suppl):9503. [Google Scholar]
- 38.Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non–small cell lung cancer treated with pembrolizumab: results from Phase I KEYNOTE-001 study. J Clin Oncol. 2019;JCO1900934. doi: 10.1200/JCO.19.00934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36(17):1668-1674. doi: 10.1200/JCO.2017.75.6270 [DOI] [PubMed] [Google Scholar]
- 40.Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582-588. doi: 10.1093/annonc/mdz011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Patient Characteristics (N = 270)
eTable 2. Univariate Analysis of Associations Between Baseline Demographic or Clinical Characteristics and Overall Survival at 5 Years in 270 patients With Melanoma, RCC, or NSCLC Receiving Nivolumab
eTable 3. Association Between Baseline Sum of Target Lesion Diameters and 5-Year Survival in All Patients Receiving Nivolumab (N = 270)
eTable 4. Association Between Objective Response and 5-Year Survival in Patients With Melanoma, RCC, or NSCLC Receiving Nivolumab (N = 270)
eTable 5. Association Between Objective Response and 5-Year Survival in all Patients Receiving Nivolumab (N = 270)
eTable 6. Summary of Treatment-related Adverse Events in All Patients Receiving Nivolumab (N = 270)
eFigure 1. Overall Survival In Patients With Melanoma, RCC, or NSCLC Based on Nivolumab Dose Received
eFigure 2. Swimmer Plots for Responders With Advanced Melanoma, RCC, or NSCLC
eFigure 3. Association Between Incidence of Treatment-Related Adverse Events and Clinical Outcomes in Patients With Melanoma, RCC, or NSCLC Receiving Nivolumab
eFigure 4. Association Between Baseline Immune Cell Counts and Objective Response in Patients With Melanoma, RCC, or NSCLC Receiving Nivolumab
eFigure 5. Association Between Baseline Immune Cell Counts and 5-Year Survival in Patients With Melanoma, RCC, or NSCLC Receiving Nivolumab
eFigure 6. Maximum Change in Tumor Burden in 23 Patients With Melanoma, RCC, or NSCLC Who Were Treated With Nivolumab Beyond Tumor Progression