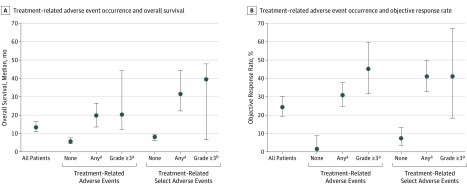
Figure 4. Association Between Incidence of Treatment-Related Adverse Events and Clinical Outcomes in 270 Patients With Melanoma, RCC, or NSCLC Receiving Nivolumab.
Analysis includes all treatment-related adverse events occurring between administration of the first dose of nivolumab and 30 days after the last dose. Incidence of treatment-related adverse events was not controlled for drug exposure. Treatment-related select adverse events are defined as those with a potential immune-mediated causality.8 P values were determined using a Cox proportional hazards regression model and are based on the hazard ratio for survival (none vs any or grade ≥3) subgroups. Error bars represent 95% CIs.
aP < .001.
bP < .05 vs none in the respective treatment-related adverse event category.