Fig. 1.
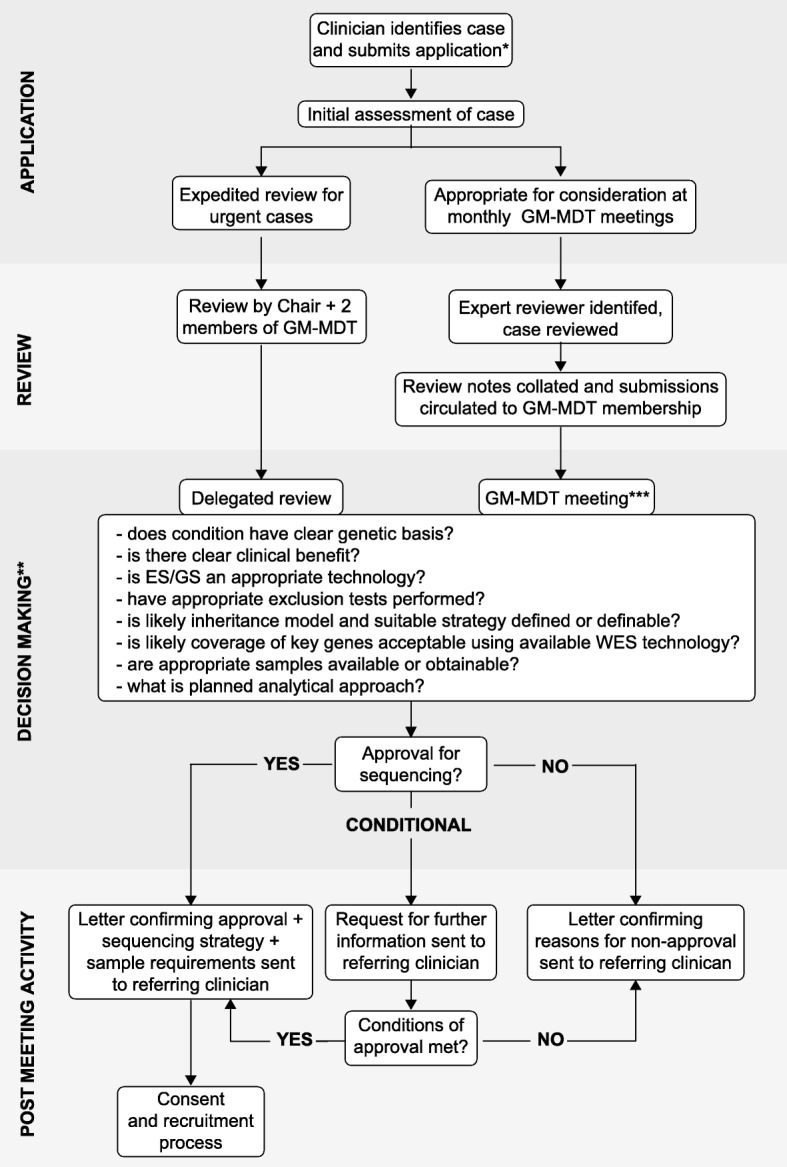
Case review and approval process for GM-MDT. *Application includes clinical phenotype and disease information, demographics, family history including pedigree, ethnicity, evidence or likelihood of consanguinity, prior genetic testing, likely clinical utility/impact on management, genes/variants known to cause the disorder, samples availability and those proposed for genetic testing. **Key questions addressed as part of review process are illustrated; other points often case specific. ***Discussion recorded by project manager in meeting minutes. Figure is based on practice up to the end of October 2015 (including all cases reported here); current process described in Ormondroyd et al. 2017 [10]
