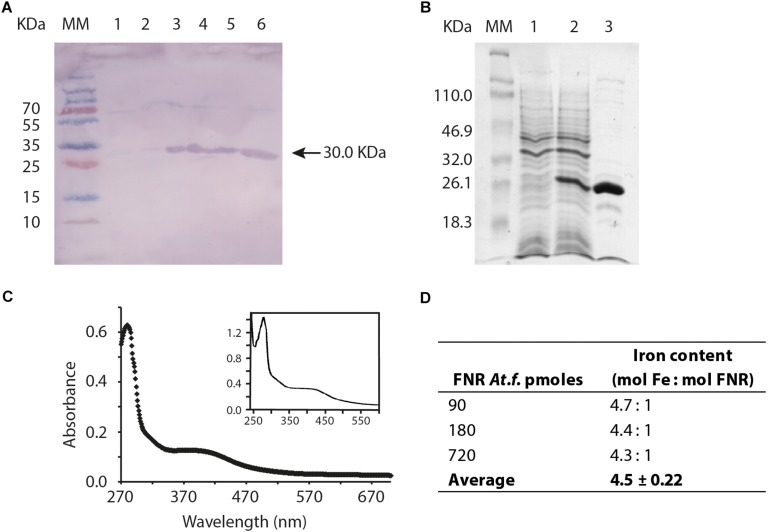
FIGURE 5.
Biochemical validation and properties of FNRAF. (A) Western blot, antibodies prepared against FNREC were used to detect FNRAF, which was overproduced from plasmid pET100/D-TOPO fnrAF cloned into E. coli strain BL21. The gel shows the molecular mass marker (MM), crude cell free extract (approximately 10 μg of total protein) prepared prior to induction of fnrAF expression with IPTG (lane 1), 10 μg crude cell free extract 1 h after induction of fnrAF expression with IPTG (lane 2), and elution’s 1 to 4 of 10 μg purified FNRAF protein after elution from the nickel column (lanes 3–6). (B) Overproduction and purification of FNRAF from the pET11A fnrAF plasmid as shown by SDS-PAGE analysis. Gel lane 1 shows crude cell free extract (approximately 10 μg of total protein) prepared prior to induction of fnrAF expression with IPTG; lane 2, 10 μg of crude cell free extract 1 h after induction of fnrAF expression with IPTG; and lane 3, 10 μg FNRAF after the second cationic interchange chromatographic column. M, molecular mass marker (Mr are indicated). (C) Ultraviolet/visible absorption spectrum of a representative result of biological duplicates for purified FNRAF from anaerobically grown At. ferrooxidans with an inset of the ultraviolet/visible spectrum of anaerobically purified FNREC taken from Lazazzera et al. (1993). (D) Calculation of the FNRAF iron content by spectrophotometric assay.