Abstract
Objectives:
Recent preclinical research has renewed interest in the interplay between glucose dysregulation and cancer. Metformin holds promise as an adjunctive antineoplastic agent in head and neck cancer (HNC). We aimed to explore the impact of metformin in HNC patients from a population-based dataset.
Patients & Methods:
Patients diagnosed with HNC from 2008 to 2011 were identified from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked dataset and categorized into three groups: non-diabetics (nD), diabetics not taking metformin (DnM), and diabetics taking metformin (D + M). Overall survival (OS) and cancer-specific survival (CSS) were compared between groups using Kaplan-Meier and Cox regression controlling for sociodemographic, clinical, and treatment covariates. The incidence of toxicities associated with HNC therapy was compared among groups using χ2 analysis.
Results:
Among 1646 patients, there were 1144 nD, 378 DnM, and 124 D + M. 2-year OS rates was 65.6% for nD, 57.7% for DnM, and 73.4% for D + M by Kaplan-Meier (p < 0.01), and corresponding rates of 2-year CSS were 73.7%, 66.1%, and 88.8% (p < 0.01), respectively. On Cox multivariable analysis, OS among the three groups did not significantly differ; however, CSS was significantly worse among both nD versus DnM as compared to D + M. Toxicity rates were not significantly increased among D + M.
Conclusion:
HNC patients with diabetes taking metformin experience improved CSS. Prospective investigation of the addition of metformin to standard-of-care HNC therapy is warranted.
Keywords: Metformin, Diabetes, Head and neck cancer, Warburg effect
Introduction
The past decade has witnessed both a growing prevalence of head and neck cancer (HNC) driven by the human papillomavirus (HPV) [1,2] and a series of innovations [3–7] that have enhanced the therapeutic ratio in this disease; however, survival outcomes in the United States remain suboptimal. At a national level, 5-year relative survival rates from cancers of the oral cavity, pharynx, and larynx remain at or below 66% [8], while even on recent prospective clinical trials, patients with advanced HNC experience 3-year overall survival (OS) and progression-free survival rates that fail to exceed 76% and 62%, respectively [9]. Novel therapeutic approaches will therefore prove essential to improving outcomes in HNC.
Building on a hypothesis initially formulated half a century ago [10], recent preclinical data have implicated dysregulation of glucose metabolism in stimulating tumor growth and proliferation [11]. It follows that disorders of glucose homeostasis, chiefly diabetes mellitus, may adversely affect outcomes in cancer patients. Clinical data support this hypothesis and suggest that premorbid diabetes confers a worse prognosis in the setting of a new diagnosis of cancer [12].
These data give rise to the possibility of using antihyperglycemic agents to improve outcomes in HNC. Metformin, a biguanide agent, has generated particular interest due to preclinical studies demonstrating its activity against multiple oncogenic pathways [13,14]. Retrospective analyses have demonstrated improved outcomes in cancer patients with diabetes taking metformin across a variety of disease sites including breast, prostate, lung, colorectum, uterus, and pancreas [15–21]. Recently, attention has also turned to the application of metformin in HNC [22]. However, data regarding its impact in this disease are conflicting, with some studies suggesting no effect of metformin on recurrence, survival, or second malignancy [23], and others demonstrating oncologic outcomes among diabetic patients taking metformin (D + M) that are not only superior to those with diabetes not taking metformin (DnM) but also comparable to those of non-diabetic patients (nD) [24,25]. These latter studies are limited, however, by their focus on single disease sites (larynx and oropharynx), study populations drawn from one or two institutions, and in the case of one series, uniform treatment approach with organ preservation.
We therefore aimed to evaluate the survival impact of metformin and associated toxicity in a national cohort of HNC patients managed with a variety of approaches by comparing recent outcomes among nD, DnM, and D + M patients using the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked dataset.
Patients and methods
Data source
The linked SEER-Medicare dataset combines two data sources. SEER uses population-based cancer registries to collect information on cancer cases occurring in approximately 28% of the U.S. population [26], including demographics, tumor characteristics, treatment, census tract-level socioeconomic measures, mortality, and cause of death. Medicare claims provide longitudinal data on diagnoses, procedures, and prescription drugs. Diagnoses and procedures are reported using the International Classification of Diseases, Ninth Revision (ICD-9), Clinical Modification codes, Current Procedural Terminology (CPT) codes, and Healthcare Common Procedure Coding System (HCPCS). In 2006, Medicare initiated the optional Part D drug benefit, which covers outpatient prescription drugs to supplement traditional Medicare and Medicare Advantage plans [27]. Part D claims are filed for each event in which a prescription is filled and include the date of service.
Sample selection
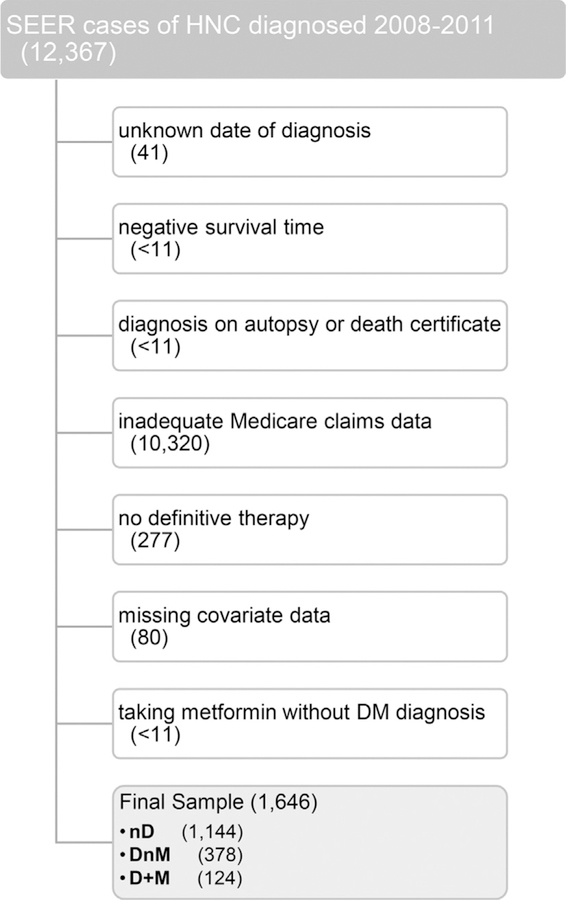
From our initial cohort of cancers of the head and neck diagnosed through 2013, we selected patients whose first and only primary tumor was a non-metastatic squamous cell carcinoma (International Classification of Diseases for Oncology ICD-O-3 morphology codes 8050–8089) of the head and neck (ICD-O-3 topography codes C00-C14) diagnosed from 2008 through 2011 (n = 12,367). We selected 2008 as the earliest year of diagnosis to ensure that at least one full year of Part D data was available prior to diagnosis (without using data from 2006, the initial year of the program), and 2011 was selected as the latest year of diagnosis to ensure most patients would have 24 months of follow-up. Patients with unknown diagnosis dates (n = 41), negative survival time (n < 11), or diagnoses identified by autopsy or death certificate (n < 11) were excluded. To capture patients with complete Medicare data, we included only beneficiaries who were least 66 years old at diagnosis and continuously enrolled in fee-for-service Medicare Parts A, B, and D for both 12 months before and 12 months following the month of diagnosis (or until death if within 12 months of diagnosis). Patients with no paid claims during the 12-month observation period were excluded (n < 11), leaving 2003 patients with complete claims data to examine prior health status, treatment, and outcomes of interest.
We used CPT, HCPCS, ICD-9, and NDC codes reported in the Medicare Provider Analysis and Review (MEDPAR), Outpatient, National Claims History (NCH) Physician/Supplier, Durable Medical Equipment (DME), and Part D (PDESAF) claims to identify patients undergoing definitive-intent therapy, which we defined as surgery, radiotherapy, or chemotherapy initiated within six months after diagnosis (n = 1726). Lastly, we excluded patients with unknown race, unknown census tract, unknown nodal stage, or nodal stage of “Not Applicable” (n = 80), leaving a sample of 1648 (Fig. 1).
Fig. 1.
Cohort Derivation. Abbreviations: SEER, Surveillance, Epidemiology, and End Results; HNC, head and neck cancer; DM, diabetes mellitus; nD, non-diabetic; DnM, diabetic not taking metformin; D + M diabetic taking metformin.
Outcomes
The initial analysis examined the characteristics associated with diabetes and metformin. Our primary focus centered on diabetes status and metformin use; however, we also incorporated statin usage and dyslipidemia status as covariates in light of recent Danish data [28], in addition to angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ACEi/ARB) and comorbid hypertension or chronic kidney disease. Prescription drugs were identified using generic names, brand names, and NDC codes on PEDSAF claims. Medication use was defined as having three or more prescriptions filled in the 12 months prior to diagnosis and three or more prescriptions filled in the year since diagnosis, unless a patient died less than a year from diagnosis, in which case we required at least one prescription filled for every four months of survival. The associated chronic conditions were identified using the ICD-9 diagnosis codes used in the Chronic Conditions Data Warehouse algorithms [29]. We considered a patient with at least one diagnosis reported on a MEDPAR, outpatient, or NCH claim in the year prior to diagnosis to have the condition. We classified patients with respect to diabetes status and metformin usage into three categories: (1) negative for diabetes and negative for metformin, i.e. nD; (2) positive for diabetes and negative for metformin, i.e. DnM; and (3) positive for diabetes and positive for metformin, i.e. D + M. As few patients were negative for diabetes and positive for metformin, these 2 patients were dropped from the sample. In total, 1646 patients met final inclusion criteria, including 1144 (69.5%) nD, 378 (23.0%) DnM, and 124 (7.5%) D+ M.
The primary outcome of interest was two-year OS, measured as the number of months from diagnosis until death due to any cause, with patients surviving more than two years censored after 24 months. As Medicare-reported death data are more robust than those reported in SEER, we used the former to assess OS through 2011. However, only SEER captures data regarding cancer-specific survival (CSS). For this outcome, patients dying of causes other than cancer were censored at the time of death. Due to the limited time for follow-up, patients diagnosed in 2011 were excluded from cancer-specific survival analyses.
Toxicity events and conditions associated with HNC therapy and occurring within six months of the initiation of any definitive treatment were examined as a secondary outcome. Specific events were identified using claim procedure and diagnosis codes. Events included gastrostomy or feeding tube placement, tracheostomy or airway obstruction, weight loss, antiemetic prescription, dysphagia, speech pathology, or any emergency department visit. We also included hospital or emergency department visits with symptoms of dehydration, malnutrition, or nausea/emesis as toxicity events.
Control variables
In all multivariate analyses, we adjusted for patient gender, age, race, marital status, SEER registry, population density (metropolitan, urban, and rural), census tract percentage of population with high school education only, census tract percentage of population below poverty, primary tumor site, and AJCC T and N categories. Using Medicare claims, we also identified and controlled for whether the primary treatment facility was a teaching hospital. To address potential differences in overall health, Medicare claims from the year prior to diagnosis were used to calculate Charlson Comorbidity Index (CCI) values according to the National Cancer Institute adaptation of the algorithm described by Klabunde et al. [30].
Statistical analysis
All statistical analyses were performed using SPSS V24.0 (SPSS Inc., Chicago, IL).
Conditions and medications.
Pearson chi-square tests were used to assess univariate associations between categorical variables and diabetes/metformin categories.
Toxicity.
We report results as predicted marginals, which are calculated by averaging the estimated probabilities of toxicity for a standardized set of patient covariates [31]. Predicted marginals standardize outcomes to the entire study sample for covariance imbalance [31,32] and can be interpreted as percentages for logistic models and means for linear models. Statistically significant trends were determined using the Wald test at p < 0.05.
Survival.
OS and CSS were first examined using the Kaplan Meier method. Univariate survival analysis was performed with the log-rank test and unadjusted Cox proportional hazards models to estimate hazard ratios (HR) with corresponding 95% confidence intervals (95%CI). Cox Proportional Hazard regression analysis was used to estimate survival, evaluated at a significance level of p < 0.05. The proportional hazards assumption was assessed using a test of Schoenfeld residuals for covariates in all final models and returned no significant results [33].
Results
Among the 1646 patients meeting inclusion criteria, 1144 (69.5%) were nD, 378 (23.0%) were DnM, and 124 (7.5%) were D + M (Table 1). Most patients were male, white non-Hispanic, and non-married, and a majority resided in metropolitan areas. Most had oral cavity as their primary site, N0 disease, and comorbidity scores of 0. A majority of patients received surgery (59.1%) and radiotherapy (64.9%), but most (54.8%) did not receive chemotherapy.
Table 1.
Patient Characteristics (χ2).
| All Patients | nD | DnM | D + M |
p
(D + M v nD) |
p
(D + M v DnM) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | n | % | ||||
| All Patients | – | 1646 | 100.0 | 1144 | 100.00 | 378 | 100.0 | 124 | 100.0 | . | . |
| Age | 66–69 | 400 | 24.3 | 286 | 25.0 | 84 | 22.2 | 30 | 24.2 | 0.85 | 0.84 |
| 70–74 | 455 | 27.6 | 325 | 28.4 | 97 | 25.7 | 33 | 26.6 | . | . | |
| 75+ | 791 | 48.1 | 533 | 46.6 | 197 | 52.1 | 61 | 49.2 | . | . | |
| Sex | Female | 682 | 41.4 | 490 | 42.8 | 150 | 39.7 | 42 | 33.9 | 0.05 | 0.25 |
| Male | 964 | 58.6 | 654 | 57.2 | 228 | 60.3 | 82 | 66.1 | . | . | |
| Race | White Non-Hispanic | 1318 | 80.1 | 949 | 83.0 | 279 | 73.8 | 90 | 72.6 | < 0.01 | 0.79 |
| Non-White or Hispanic | 328 | 19.9 | 195 | 17.1 | 99 | 26.2 | 34 | 27.4 | . | . | |
| Marital Status | Non-Married | 892 | 54.2 | 619 | 54.1 | 218 | 57.7 | 55 | 44.4 | 0.04 | 0.01 |
| Married | 754 | 45.8 | 525 | 45.9 | 160 | 42.3 | 69 | 55.7 | . | . | |
| Geographic Region | East | 277 | 16.8 | 175 | 15.3 | 77 | 20.4 | 25 | 20.2 | 0.46 | 0.65 |
| Midwest | 208 | 12.6 | 143 | 12.5 | 53 | 14.0 | 12 | 9.7 | . | . | |
| South | 452 | 27.5 | 316 | 27.6 | 101 | 26.7 | 35 | 28.2 | . | . | |
| West | 709 | 43.1 | 510 | 44.6 | 147 | 38.9 | 52 | 41.9 | . | . | |
| Population Density | Metropolitan | 1305 | 79.3 | 897 | 78.4 | 301 | 79.6 | 107 | 86.3 | 0.04 | 0.10 |
| Non-Metropolitan | 341 | 20.7 | 247 | 21.6 | 77 | 20.4 | 17 | 13.7 | . | . | |
| Year of Diagnosis | 2008 | 382 | 23.2 | 264 | 23.1 | 89 | 23.5 | 29 | 23.4 | 0.84 | 0.55 |
| 2009 | 394 | 23.9 | 269 | 23.5 | 96 | 25.4 | 29 | 23.4 | . | . | |
| 2010 | 394 | 23.9 | 279 | 24.4 | 81 | 21.4 | 34 | 27.4 | . | . | |
| 2011 | 476 | 28.9 | 332 | 29.0 | 112 | 29.6 | 32 | 25.8 | . | . | |
| Primary Site | Oral Cavity | 835 | 50.7 | 583 | 51.0 | 180 | 47.6 | 72 | 58.1 | 0.29 | 0.05 |
| Oropharynx | 585 | 35.5 | 397 | 34.7 | 153 | 40.5 | 35 | 28.2 | . | . | |
| Other | 226 | 13.7 | 164 | 14.3 | 45 | 11.9 | 17 | 13.7 | . | . | |
| T-Classification | T0–1 | 472 | 28.7 | 344 | 30.1 | 95 | 25.1 | 33 | 26.6 | 0.15 | 0.57 |
| T2 | 489 | 29.7 | 330 | 28.9 | 118 | 31.2 | 41 | 33.1 | . | . | |
| T3–4 | 467 | 28.4 | 330 | 28.9 | 109 | 28.8 | 28 | 22.6 | . | . | |
| Unknown | 218 | 13.2 | 140 | 12.2 | 56 | 14.8 | 22 | 17.7 | . | . | |
| N-Classification | N0 | 910 | 55.3 | 625 | 54.6 | 211 | 55.8 | 74 | 59.7 | 0.41 | 0.52 |
| N1 | 281 | 17.1 | 197 | 17.2 | 62 | 16.4 | 22 | 17.7 | . | . | |
| N2–3 | 455 | 27.6 | 322 | 28.2 | 105 | 27.8 | 28 | 22.6 | . | . | |
| Comorbidity Index | 0 | 1037 | 63.0 | 766 | 67.0 | 191 | 50.5 | 80 | 64.5 | 0.58 | 0.01 |
| 1+ | 609 | 37.0 | 378 | 33.0 | 187 | 49.5 | 44 | 35.5 | . | . | |
| Teaching Hospital | No or Unknown | 748 | 45.4 | 519 | 45.4 | 178 | 47.1 | 51 | 41.1 | 0.37 | 0.25 |
| Yes | 898 | 54.6 | 625 | 54.6 | 200 | 52.9 | 73 | 58.9 | . | . | |
| Tract % High School Only | ≤ Median | 823 | 50.0 | 583 | 51.0 | 174 | 46.0 | 66 | 53.2 | 0.63 | 0.16 |
| > Median | 823 | 50.0 | 561 | 49.0 | 204 | 54.0 | 58 | 46.8 | . | . | |
| Tract % Below Poverty | ≤ Median | 824 | 50.1 | 587 | 51.3 | 169 | 44.7 | 68 | 54.8 | 0.46 | 0.05 |
| > Median | 822 | 49.9 | 557 | 48.7 | 209 | 55.3 | 56 | 45.2 | . | . | |
| Surgery | No | 673 | 40.9 | 459 | 40.1 | 164 | 43.4 | 50 | 40.3 | 0.97 | 0.55 |
| Yes | 973 | 59.1 | 685 | 59.9 | 214 | 56.6 | 74 | 59.7 | . | . | |
| Chemotherapy | No | 902 | 54.8 | 632 | 55.2 | 201 | 53.2 | 69 | 55.7 | 0.93 | 0.63 |
| Yes | 744 | 45.2 | 512 | 44.8 | 177 | 46.8 | 55 | 44.4 | . | . | |
| Radiotherapy | No | 578 | 35.1 | 408 | 35.7 | 124 | 32.8 | 46 | 37.1 | 0.75 | 0.38 |
| Yes | 1068 | 64.9 | 736 | 64.3 | 254 | 67.2 | 78 | 62.9 | . | . | |
| ACEi/ARB | No | 1069 | 65.0 | 794 | 69.4 | 233 | 61.6 | 42 | 33.9 | < 0.01 | < 0.001 |
| Yes | 577 | 35.1 | 350 | 30.6 | 145 | 38.4 | 82 | 66.1 | . | . | |
| Statin | No | 1061 | 64.5 | 795 | 69.5 | 225 | 59.5 | 41 | 33.1 | < 0.01 | < 0.01 |
| Yes | 585 | 35.5 | 349 | 30.5 | 153 | 40.5 | 83 | 66.9 | . | . | |
| HTN/CKDa | No | < 398 | < 24.2 | 343 | 30.0 | 44 | 11.6 | < 11 | < 8.9 | < 0.01 | 0.06 |
| Yes | > 1248 | > 75.8 | 801 | 70.0 | 334 | 88.4 | > 113 | > 91.1 | . | . | |
| HLD | No | 550 | 33.4 | 451 | 39.4 | 78 | 20.6 | 21 | 16.9 | < 0.01 | 0.37 |
| Yes | 1096 | 66.6 | 693 | 60.6 | 300 | 79.4 | 103 | 83.1 | . | . | |
Abbreviations: NH, non-Hispanic; nD, non-diabetic; DnM, diabetic not taking metformin; D + M diabetic taking metformin; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotension II receptor blocker; HTN, hypertension; CKD, chronic kidney disease; HLD, hyperlipidemia;
Data coarsened to comply with CMS cell size suppression policy.
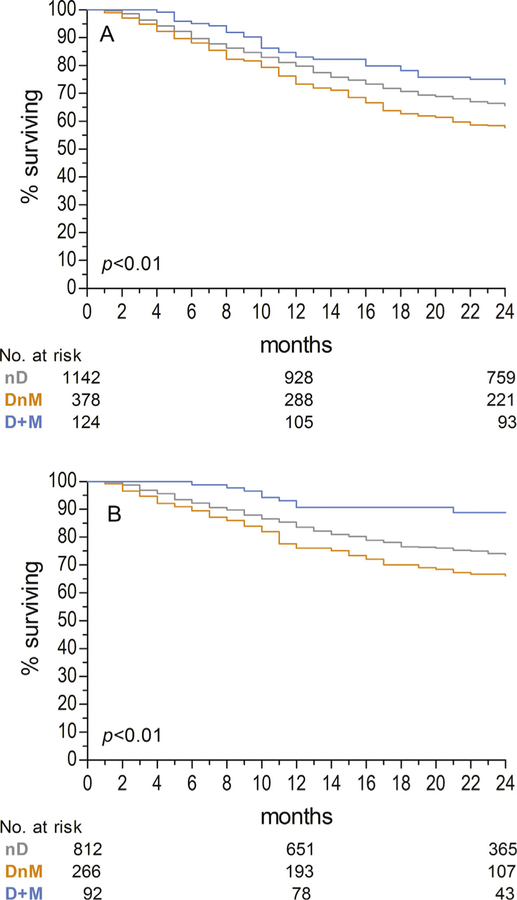
Across diabetes/metformin groups, 2-year rates of OS were lowest among DnM patients at 57.7% and highest among D + M at 73.4%, with nD patients falling in between at 65.6% (p < 0.01). Similarly, rates of CSS at 2 years were lowest among DnM patients at 66.1% and highest among D + M at 88.8%, with nD patients again falling in between at 73.7% (p < 0.01). Kaplan-Meier curves for OS and CSS are depicted in Fig. 2A and B, respectively.
Fig. 2.
(A) Overall Survival and (B) Cancer-Specific Survival. Abbreviations: nD, non-diabetic; DnM, diabetic not taking metformin; D + M diabetic taking metformin.
In multivariate Cox proportional hazard regression for OS (Table 2), neither non-metformin group experienced significantly different OS from the D + M group, although there was a trend toward worse survival in the DnM group (nD: HR 1.13, 95%CI 0.78–1.65, p = 0.53; DnM: HR 1.36, 95% CI 0.92–2.00, p = 0.12). However, statin usage, receipt of care at a teaching hospital, surgery, and radiotherapy were each associated with improved OS, while age ≥75, non-married status, increasing T-and N-classification, comorbidity score ≥1, and poorer census tract were each associated with worse OS.
Table 2.
Overall Survival.
| Kaplan-Meier | Cox UVA | Cox MVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS2 (%) | 95%CI (%) | p | HR | 95%CI | p (class) | HR | 95%CI | p (class) | p (variable) | ||
| Diabetes/Metformin Category | D + M | 73.4 | 64.7–80.3 | < 0.01 | . | . | . | . | . | . | 0.11 |
| nD | 65.6 | 62.8–68.3 | . | 1.37 | 0.96–1.96 | 0.08 | 1.13 | 0.78–1.65 | 0.53 | . | |
| DnM | 57.7 | 52.5–62.5 | . | 1.79 | 1.23–2.60 | < 0.01 | 1.36 | 0.92–2.00 | 0.12 | . | |
| Age | 66–69 | 72.5 | 67.8–76.6 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| 70–74 | 73.6 | 69.3–77.4 | . | 0.96 | 0.74–1.24 | 0.75 | 1.06 | 0.82–1.38 | 0.65 | . | |
| 75+ | 55.0 | 51.5–58.4 | . | 1.87 | 1.51–2.32 | < 0.01 | 2.06 | 1.64–2.59 | < 0.01 | . | |
| Sex | Male | 66.0 | 62.9–68.9 | 0.13 | . | . | . | . | . | . | 0.43 |
| Female | 62.2 | 58.4–65.7 | . | 1.13 | 0.96–1.33 | 0.13 | 1.08 | 0.90–1.29 | 0.43 | . | |
| Race/Ethnicity | White Non-Hispanic | 65.1 | 62.5–67.6 | 0.30 | . | . | . | . | . | . | 0.40 |
| Non-White or Hispanic | 61.6 | 56.1–66.6 | . | 1.11 | 0.91–1.35 | 0.30 | 0.91 | 0.73–1.13 | 0.40 | . | |
| Marital Status | Married | 72.7 | 69.3–75.7 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| Non-Married | 57.4 | 54.1–60.6 | . | 1.74 | 1.47–2.06 | < 0.01 | 1.43 | 1.19–1.72 | < 0.01 | . | |
| Geographic Region | West | 64.0 | 60.4–67.4 | 0.40 | . | . | . | . | . | . | 0.48 |
| East | 66.1 | 60.2–71.3 | . | 0.94 | 0.74–1.19 | 0.63 | 0.81 | 0.62–1.07 | 0.14 | . | |
| Midwest | 68.8 | 62.0–74.6 | . | 0.86 | 0.66–1.13 | 0.29 | 0.88 | 0.64–1.21 | 0.43 | . | |
| South | 61.9 | 57.3–66.2 | . | 1.08 | 0.89–1.32 | 0.41 | 0.88 | 0.70–1.11 | 0.28 | . | |
| Population Density | Metropolitan | 64.8 | 62.2–67.4 | 0.35 | . | . | . | . | . | . | 0.67 |
| Non-Metropolitan | 62.8 | 57.4–67.6 | . | 1.10 | 0.90–1.33 | 0.36 | 0.95 | 0.75–1.20 | 0.67 | . | |
| Year of Diagnosis | 2008 | 62.8 | 57.8–67.5 | 0.64 | . | . | . | . | . | . | 0.37 |
| 2009 | 64.0 | 59.0–68.5 | . | 0.95 | 0.75–1.20 | 0.67 | 0.94 | 0.74–1.19 | 0.60 | . | |
| 2010 | 63.7 | 58.7–68.2 | . | 0.97 | 0.77–1.22 | 0.79 | 1.00 | 0.79–1.26 | 0.97 | . | |
| 2011 | 66.6 | 62.2–70.6 | . | 0.87 | 0.69–1.09 | 0.23 | 0.83 | 0.66–1.05 | 0.12 | . | |
| Primary Site | Oral Cavity | 68.1 | 64.9–71.2 | < 0.01 | . | . | . | . | . | . | 0.06 |
| Oropharynx | 62.4 | 58.3–66.2 | . | 1.24 | 1.04–1.49 | 0.02 | 0.75 | 0.60–0.95 | 0.02 | . | |
| Other | 55.8 | 49.0–61.9 | . | 1.54 | 1.23–1.94 | < 0.01 | 0.87 | 0.67–1.13 | 0.29 | . | |
| T-Classification | T0–1 | 80.3 | 76.4–83.6 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| T2 | 64.0 | 59.6–68.1 | . | 2.00 | 1.55–2.57 | < 0.01 | 2.00 | 1.10–3.64 | 0.02 | . | |
| T3–4 | 47.3 | 42.7–51.8 | . | 3.48 | 2.74–4.42 | < 0.01 | 5.67 | 3.21–10.0 | < 0.01 | . | |
| Unknown | 67.4 | 60.8–73.2 | . | 1.88 | 1.38–2.55 | < 0.01 | 4.31 | 2.22–8.37 | < 0.01 | . | |
| N-Classification | N0 | 71.8 | 68.7–74.6 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| N1 | 56.2 | 50.2–61.8 | . | 1.72 | 1.39–2.14 | < 0.01 | 1.58 | 1.24–2.01 | < 0.01 | . | |
| N2–3 | 54.7 | 50.0–59.2 | . | 1.80 | 1.50–2.17 | < 0.01 | 1.78 | 1.42–2.25 | < 0.01 | . | |
| Comorbidity Index | 0 | 70.5 | 67.6–73.2 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| 1+ | 54.0 | 50.0–57.9 | . | 1.80 | 1.53–2.12 | < 0.01 | 1.69 | 1.42–2.01 | < 0.01 | . | |
| Tract % High School Only | ≤ Median | 66.8 | 63.5–69.9 | 0.02 | . | . | . | . | . | . | 0.02 |
| > Median | 62.0 | 58.6–65.2 | . | 1.21 | 1.02–1.42 | 0.02 | 1.29 | 1.05–1.59 | 0.02 | . | |
| Tract % Below Poverty | ≤ Median | 68.2 | 64.9–71.3 | < 0.01 | . | . | . | . | . | . | 0.45 |
| > Median | 60.6 | 57.2–63.8 | . | 1.32 | 1.12–1.55 | < 0.01 | 1.08 | 0.89–1.30 | 0.45 | . | |
| Teaching Hospital | No or Unknown | 61.1 | 57.5–64.5 | 0.01 | . | . | . | . | . | . | 0.02 |
| Yes | 67.1 | 64.0–70.1 | . | 0.80 | 0.68–0.95 | 0.01 | 0.81 | 0.68–0.96 | 0.02 | . | |
| Surgery | No | 51.9 | 48.0–55.6 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| Yes | 73.1 | 70.2–75.7 | . | 0.48 | 0.40–0.56 | < 0.01 | 0.54 | 0.43–0.67 | < 0.01 | . | |
| Chemotherapy | No | 69.0 | 65.8–71.9 | < 0.01 | . | . | . | . | . | . | 0.35 |
| Yes | 58.9 | 55.2–62.3 | . | 1.40 | 1.19–1.64 | < 0.01 | 0.90 | 0.72–1.12 | 0.35 | . | |
| Radiotherapy | No | 74.2 | 70.5–77.6 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| Yes | 59.1 | 56.1–62.0 | . | 1.70 | 1.41–2.04 | < 0.01 | 0.43 | 0.28–0.65 | < 0.01 | . | |
| ACEi or ARB | No | 61.2 | 58.2–64.0 | < 0.01 | . | . | . | . | . | . | 0.51 |
| Yes | 70.4 | 66.5–73.9 | . | 0.73 | 0.61–0.88 | < 0.01 | 1.13 | 0.78–1.65 | 0.51 | . | |
| Statin | No | 60.3 | 57.3–63.2 | < 0.01 | . | . | . | . | . | . | 0.01 |
| Yes | 71.8 | 68.0–75.3 | . | 0.66 | 0.55–0.79 | < 0.01 | 0.75 | 0.62–0.93 | 0.01 | . | |
| HTN/CKD | No | 67.8 | 62.9–72.1 | 0.07 | . | . | . | . | . | . | 0.15 |
| Yes | 63.3 | 60.6–65.9 | . | 1.20 | 0.98–1.46 | 0.07 | 1.18 | 0.94–1.48 | 0.15 | . | |
| HLD | No | 58.7 | 54.5–62.7 | < 0.01 | . | . | . | . | . | . | 0.05 |
| Yes | 67.2 | 64.4–69.9 | . | 0.75 | 0.63–0.88 | < 0.01 | 0.82 | 0.68–1.00 | 0.05 | . | |
Abbreviations: OS2, 2-year overall survival; 95%CI, 95% confidence interval; HR, hazard ratio; UVA, univariate analysis; MVA, multivariate analysis; nD, non-diabetic; DnM, diabetic not taking metformin; D + M diabetic taking metformin; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotension II receptor blocker; HTN, hypertension; CKD, chronic kidney disease; HLD, hyperlipidemia.
On multivariate Cox regression for CSS (Table 3), both nD and DnM experienced significantly worse CSS as compared D + M (nD: HR 2.33, 95%CI 1.16–4.65, p = 0.02; DnM: HR 3.03, 95% CI 1.49–6.16, p < 0.01). Improved CSS was associated with oropharyngeal primary site and surgery, while age ≥75, female sex, increasing T-and N-classification, comorbidity score ≥1, and poorer census tract were each associated with worse CSS.
Table 3.
Cancer-Specific Survival.
| Kaplan-Meier | Cox UVA | Cox MVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSS2 (%) | 95%CI (%) | p | HR | 95%CI | p (class) | HR | 95%CI | p (class) | p (variable) | ||
| Diabetes/Metformin Category | D + M | 88.8 | 79.2–94.1 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| nD | 73.7 | 70.3–76.8 | . | 2.54 | 1.30–4.95 | 0.01 | 2.33 | 1.16–4.65 | 0.02 | . | |
| DnM | 66.1 | 59.6–71.8 | . | 3.50 | 1.76–6.97 | < 0.01 | 3.03 | 1.49–6.16 | < 0.01 | . | |
| Age | 66–69 | 77.6 | 71.9–82.2 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| 70–74 | 78.0 | 72.8–82.3 | . | 0.99 | 0.70–1.40 | 0.95 | 1.04 | 0.73–1.50 | 0.82 | . | |
| 75+ | 67.7 | 63.2–71.7 | . | 1.56 | 1.16–2.10 | < 0.01 | 1.77 | 1.29–2.44 | < 0.01 | . | |
| Sex | Male | 76.6 | 72.9–79.9 | 0.01 | . | . | . | . | . | . | < 0.01 |
| Female | 68.6 | 64.0–72.7 | . | 1.38 | 1.09–1.74 | 0.01 | 1.55 | 1.19–2.02 | < 0.01 | . | |
| Race/Ethnicity | White Non-Hispanic | 74.8 | 71.7–77.7 | 0.04 | . | . | . | . | . | . | 0.77 |
| Non-White or Hispanic | 66.1 | 59.0–72.4 | . | 1.33 | 1.01–1.74 | 0.04 | 1.05 | 0.77–1.42 | 0.77 | . | |
| Marital Status | Married | 79.8 | 75.8–83.1 | < 0.01 | . | . | . | . | . | . | 0.12 |
| Non-Married | 67.5 | 63.3–71.3 | . | 1.70 | 1.33–2.17 | < 0.01 | 1.24 | 0.95–1.63 | 0.12 | . | |
| Geographic Region | West | 70.7 | 66.2–74.7 | 0.08 | . | . | . | . | . | . | 0.12 |
| East | 75.6 | 68.6–81.3 | . | 0.81 | 0.58–1.14 | 0.22 | 0.68 | 0.46–1.02 | 0.06 | . | |
| Midwest | 82.6 | 74.6–88.2 | . | 0.60 | 0.38–0.94 | 0.02 | 0.60 | 0.36–1.00 | 0.05 | . | |
| South | 71.4 | 65.7–76.4 | . | 1.02 | 0.77–1.34 | 0.92 | 0.77 | 0.55–1.08 | 0.12 | . | |
| Population Density | Metropolitan | 73.5 | 70.3–76.4 | 0.41 | . | . | . | . | . | . | 0.78 |
| Non-Metropolitan | 71.7 | 65.1–77.2 | . | 1.12 | 0.85–1.49 | 0.42 | 0.95 | 0.68–1.34 | 0.78 | . | |
| Year of Diagnosis | 2008 | 73.8 | 68.9–78.1 | 0.87 | . | . | . | . | . | . | 0.90 |
| 2009 | 72.3 | 67.4–76.5 | . | 1.07 | 0.81–1.42 | 0.61 | 1.06 | 0.80–1.42 | 0.68 | . | |
| 2010 | 73.8 | 67.5–79.1 | . | 1.02 | 0.76–1.37 | 0.92 | 1.06 | 0.78–1.44 | 0.70 | . | |
| Primary Site | Oral Cavity | 77.0 | 73.1–80.4 | < 0.01 | . | . | . | . | . | . | 0.01 |
| Oropharynx | 70.5 | 65.4–75.0 | . | 1.40 | 1.08–1.82 | 0.01 | 0.62 | 0.45–0.85 | < 0.01 | . | |
| Other | 65.5 | 57.2–72.5 | . | 1.68 | 1.22–2.32 | < 0.01 | 0.71 | 0.49–1.02 | 0.06 | . | |
| T-Classification | T0–1 | 89.1 | 85.1–92.1 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| T2 | 69.8 | 63.9–74.9 | . | 2.98 | 2.01–4.42 | < 0.01 | 1.95 | 0.77–4.92 | 0.16 | . | |
| T3–4 | 56.7 | 50.6–62.3 | . | 5.36 | 3.68–7.80 | < 0.01 | 7.62 | 3.17–18.3 | < 0.01 | . | |
| Unknown | 74.6 | 66.5–81.1 | . | 2.74 | 1.73–4.36 | < 0.01 | 5.20 | 1.91–14.2 | < 0.01 | . | |
| N-Classification | N0 | 80.9 | 77.5–83.9 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| N1 | 63.7 | 55.8–70.6 | . | 2.14 | 1.57–2.91 | < 0.01 | 1.63 | 1.15–2.31 | 0.01 | . | |
| N2–3 | 62.6 | 56.6–68.0 | . | 2.21 | 1.69–2.88 | < 0.01 | 1.80 | 1.30–2.49 | < 0.01 | . | |
| Comorbidity Index | 0 | 76.1 | 72.6–79.2 | < 0.01 | . | . | . | . | . | . | 0.04 |
| 1+ | 67.8 | 62.7–72.5 | . | 1.47 | 1.16–1.86 | < 0.01 | 1.31 | 1.02–1.69 | 0.04 | . | |
| Tract % High School Only | ≤ Median | 76.0 | 75.0–79.6 | 0.01 | . | . | . | . | . | . | < 0.01 |
| > Median | 70.2 | 66.1–73.9 | . | 1.39 | 1.10–1.76 | 0.01 | 1.56 | 1.15–2.11 | < 0.01 | . | |
| Tract % Below Poverty | ≤ Median | 78.6 | 74.7–81.9 | < 0.01 | . | . | . | . | . | . | 0.24 |
| > Median | 67.7 | 63.5–71.6 | . | 1.66 | 1.30–2.10 | < 0.01 | 1.19 | 0.89–1.58 | 0.24 | . | |
| Teaching Hospital | No or Unknown | 69.4 | 65.0–73.3 | 0.01 | . | . | . | . | . | . | 0.13 |
| Yes | 76.3 | 72.5–79.7 | . | 0.72 | 0.57–0.91 | 0.01 | 0.82 | 0.64–1.06 | 0.13 | . | |
| Surgery | No | 58.6 | 53.6–63.2 | < 0.01 | . | . | . | . | . | . | < 0.01 |
| Yes | 83.0 | 79.8–85.8 | . | 0.34 | 0.26–0.43 | < 0.01 | 0.42 | 0.30–0.58 | < 0.01 | . | |
| Chemotherapy | No | 80.4 | 76.8–83.4 | < 0.01 | . | . | . | . | . | . | 0.74 |
| Yes | 64.5 | 59.9–68.7 | . | 1.92 | 1.51–2.44 | < 0.01 | 1.06 | 0.77–1.44 | 0.74 | . | |
| Radiotherapy | No | 85.4 | 81.3–88.6 | < 0.01 | . | . | . | . | . | . | 0.05 |
| Yes | 66.3 | 62.5–69.8 | . | 2.50 | 1.86–3.36 | < 0.01 | 0.53 | 0.28–1.01 | 0.05 | . | |
| ACEi or ARB | No | 71.0 | 67.3–74.3 | 0.04 | . | . | . | . | . | . | 0.52 |
| Yes | 77.0 | 72.3–81.0 | . | 0.77 | 0.60–0.99 | 0.04 | 0.91 | 0.69–1.21 | 0.52 | . | |
| Statin | No | 69.2 | 65.5–72.6 | < 0.01 | . | . | . | . | . | . | 0.09 |
| Yes | 80.1 | 75.6–83.8 | . | 0.61 | 0.47–0.80 | < 0.01 | 0.77 | 0.57–1.04 | 0.09 | . | |
| HTN/CKD | No | 75.7 | 69.9–80.5 | 0.29 | . | . | . | . | . | . | 0.21 |
| Yes | 72.3 | 68.9–75.3 | . | 1.16 | 0.88–1.54 | 0.29 | 1.23 | 0.89–1.71 | 0.21 | . | |
| HLD | No | 69.1 | 63.9–73.7 | 0.03 | . | . | . | . | . | . | 0.21 |
| Yes | 75.2 | 71.7–78.2 | . | 0.77 | 0.60–0.97 | 0.03 | 0.83 | 0.63–1.11 | 0.21 | . | |
Abbreviations: CSS2, 2-year cancer-specific survival; 95%CI, 95% confidence interval; HR, hazard ratio; UVA, univariate analysis; MVA, multivariate analysis; nD, non-diabetic; DnM, diabetic not taking metformin; D + M diabetic taking metformin; NH, non-Hispanic; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotension II receptor blocker; HTN, hypertension; CKD, chronic kidney disease; HLD, hyperlipidemia.
Toxicity outcomes are depicted in Table 4. The most common toxicity events were dysphagia (47.4%), antiemetic prescription (46.8%), gastrostomy (38.3%), and weight loss (34.5%), with the incidence of every other event falling below 30%. Toxicity rates were generally consistent across between metformin and non-metformin groups, as the incidence of each assessed toxicity did not significantly differ between them.
Table 4.
Toxicity Incidence.
| All Patients | nD | DnM | D + M |
p
(D + M v nD) |
p
(D + M v DnM) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | n | % | ||||
| All Patients | – | 1646 | 100.0 | 1144 | 100.0 | 378 | 100.0 | 124 | 100.0 | . | . |
| Weight Loss | No | 1078 | 65.5 | 764 | 66.8 | 233 | 61.6 | 81 | 65.3 | 0.74 | 0.46 |
| Yes | 568 | 34.5 | 380 | 33.2 | 145 | 38.4 | 43 | 34.7 | . | . | |
| Antiemetic Prescription | No | 875 | 53.2 | 603 | 52.7 | 209 | 55.3 | 63 | 50.8 | 0.69 | 0.38 |
| Yes | 771 | 46.8 | 541 | 47.3 | 169 | 44.7 | 61 | 49.2 | . | . | |
| ED Visit | No | 1161 | 70.5 | 818 | 71.5 | 262 | 69.3 | 81 | 65.3 | 0.15 | 0.41 |
| Yes | 485 | 29.5 | 326 | 28.5 | 116 | 30.7 | 43 | 34.7 | . | . | |
| Hospital/ED Visit w/Nausea/Emesis | No | 1360 | 82.6 | 944 | 82.5 | 312 | 82.5 | 104 | 83.9 | 0.71 | 0.73 |
| Yes | 286 | 17.4 | 200 | 17.5 | 66 | 17.5 | 20 | 16.1 | . | . | |
| Hospital/ED Visit w/Dehydration | No | 1201 | 73.0 | 834 | 72.9 | 277 | 73.3 | 90 | 72.6 | 0.94 | 0.88 |
| Yes | 445 | 27.0 | 310 | 27.1 | 101 | 26.7 | 34 | 27.4 | . | . | |
| Hospital/ED Visit w/Malnutrition | No | 1225 | 74.4 | 857 | 74.9 | 275 | 72.8 | 93 | 75.0 | 0.98 | 0.62 |
| Yes | 421 | 25.6 | 287 | 25.1 | 103 | 27.3 | 31 | 25.0 | . | . | |
| Gastrostomy or Feeding Tube | No | 1015 | 61.7 | 718 | 62.8 | 224 | 59.3 | 73 | 58.9 | 0.40 | 0.94 |
| Yes | 631 | 38.3 | 426 | 37.2 | 154 | 40.7 | 51 | 41.1 | . | . | |
| Dysphagia | No | 866 | 52.6 | 618 | 54.0 | 187 | 49.5 | 61 | 49.2 | 0.31 | 0.96 |
| Yes | 780 | 47.4 | 526 | 46.0 | 191 | 50.5 | 63 | 50.8 | . | . | |
| Tracheostomy or Airway Obstruction | No | 1225 | 74.4 | 852 | 74.5 | 274 | 72.5 | 99 | 79.8 | 0.19 | 0.10 |
| Yes | 421 | 25.6 | 292 | 25.5 | 104 | 27.5 | 25 | 20.2 | . | . | |
| Speech Pathology | No | 1343 | 81.6 | 937 | 81.9 | 305 | 80.7 | 101 | 81.5 | 0.90 | 0.85 |
| Yes | 303 | 18.4 | 207 | 18.1 | 73 | 19.3 | 23 | 18.6 | . | . | |
Abbreviations: ED, emergency department; nD, non-diabetic; DnM, diabetic not taking metformin; D + M diabetic taking metformin.
Discussion
In a nationally-representative cohort of HNC patients, we identified improved OS and CSS among D + M as compared not only to nD but also to DnM. Even after controlling for imbalanced covariates on MVA, D + M appeared to experience improved CSS as compared to DnM or nD.
It may not be surprising that patients with diabetes taking metformin (D + M experience improved outcomes as compared to their counterparts not taking metformin) [22]. After all, patients who take metformin may also be more likely to exhibit other health behaviors associated with improved outcomes in HNC, including abstinence from tobacco or alcohol and adherence to oncologist-recommended care. What is remarkable, however, is our finding that diabetic patients taking metformin experience a significant CSS advantage over patients without diabetes. This provocative result suggests that metformin may not only overcome the negative impact of diabetes in HNC, but may also confer additional anticancer benefit beyond promoting normoglycemia.
Our findings are therefore in line with the published literature suggesting that metformin exerts antitumor effects in HNC [14]. Preclinical evidence demonstrates that metformin activates adenosine monophosphate-activated protein kinase (AMPK) [34], which in turn suppresses the Warburg effect and inhibits tumor growth [35]. Via both AMPK-dependent and AMPK-independent pathways, metformin downregulates mammalian target of rapamycin (mTOR), thereby suppressing cell proliferation, growth, and survival [36]. These antineoplastic effects (and others) may underlie the improved outcomes among patients taking metformin observed in our study and others [24,25].
Intriguingly, the prolonged survival we observed among metformin users did not appear to come at the cost of increased toxicity, in contrast to previous studies [37]. Our findings are therefore consistent with metformin being well-tolerated in general. Unlike other drugs classes used in the management of diabetes, biguanides have an antihyperglycemic mechanism of action, rather than a hypoglycemic one [38], rendering hypoglycemic episodes rare. This is an important consideration in patients undergoing therapy for HNC, who tend to experience mucosal toxicity that can limit oral nutrition and may therefore be predisposed to hypoglycemia. Indeed, the most serious adverse effect of metformin is lactic acidosis, which is fortunately rare among metformin users and typically occurs in patients inappropriately selected for metformin therapy [39,40].
Our study joins others that support the repurposing of drugs traditionally used in non-cancer settings for the treatment of cancer [41]. Notably, preclinical data have demonstrated that statins exert an anticarcinogenic effect [42] (including against HNC specifically [43]), and a recent study from Denmark identified improved cancer mortality among statin users [28]. It is therefore notable that the effect of metformin in our study persisted even after adjusting for statin usage. The expanded use of typically generic (and therefore inexpensive) medications whose pharmacokinetic and pharmacodynamic properties are well-characterized holds appeal as our health care system strains under the burden of escalating costs for oncology care [44–46].
There are limitations inherent to SEER-Medicare that should be noted. First, as a retrospective analysis, our study is subject to selection bias, and it is possible that unmeasured confounders may have influenced our findings. Second, established prognostic factors such as performance status, HPV status, tobacco usage, and high-risk pathologic features are unavailable in SEER-Medicare and may have been imbalanced between diabetes/metformin groups. Third, SEER-Medicare only captures patients eligible for Medicare (typically ages 65 and up), and we only included patients enrolled on fee-for-service Medicare, which both limit the generalizability of our findings to younger patients or those on Medicare Advantage plans. Finally, oncologic outcomes from SEER-Medicare are limited to survival and cause of death. As such, we are unable to compare locoregional control, distant metastases, or disease-free survival between diabetes/metformin groups. Moreover, the particular selection criteria and covariates (including comorbidities and medications) we applied may also introduce bias beyond that specific to SEER-Medicare itself.
In light of these limitations, our study can only be considered hypothesis-generating, and prospective validation of our findings is warranted. Two prospective, single-arm trials are evaluating the addition of metformin to standard-of-care cisplatin-based chemoradiotherapy for HNC (P, ). Should these pilot trials demonstrate the safety of metformin in this setting, they will justify subsequent phase II-III trials randomizing patients to standard-of-care chemoradiation ± metformin, analogous to the cooperative group study in the realm of locoregionally-advanced non-small cell lung cancer [47] that recently met its accrual goal.
In summary, this population-based study of the effect of metformin on survival in HNC demonstrated that diabetic patients taking metformin experienced significant prolongation in cancer-specific survival and nonsignificant prolongation of overall survival as compared to both nondiabetic patients and diabetic patients not taking metformin. Moreover, metformin was not associated with excess treatment-related toxicity. These findings therefore justify current prospective investigations adding metformin to standard-of-care therapy in HNC.
Acknowledgements
This project was supported by Population Health Shared Resource, University of Colorado Cancer Center, P30CA046934. Dr. Karam is supported by the Paul Calabresi Career Development Award for Clinical Oncology (K12, CA086913), RSNA grant (#RSD1713), Golfer’s against Cancer, Cancer League of Colorado Grant, and the Marsico fund.
Footnotes
Conflict of interest statement
None declared.
References
- [1].Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol: Official J Am Soc Clin Oncol 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013;105:175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yamazaki H, Nishiyama K, Tanaka E, Koizumi M, Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol* Biol*Phys 2006;64:77–82. [DOI] [PubMed] [Google Scholar]
- [4].Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21–8. [DOI] [PubMed] [Google Scholar]
- [5].Blanchard P, Baujat B, Holostenco V, Bourredjem A, Baey C, Bourhis J, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol 2011;100:33–40. [DOI] [PubMed] [Google Scholar]
- [6].Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, et al. Long-term results of RTOG 91–11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 2013;31:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: Cancer J Clinicians 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [9].Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol: Official J Am Soc Clin Oncol 2014;32:2940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Warburg O On the origin of cancer cells. Sci (N Y, NY) 1956;123:309–14. [DOI] [PubMed] [Google Scholar]
- [11].Peeters K, Van Leemputte F, Fischer B, Bonini BM, Quezada H, Tsytlonok M, et al. Fructose-1,6-bisphosphate couples glycolytic flux to activation of Ras. Nat Commun 2017;8:922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. Jama 2008;300:2754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Del Barco S, Vazquez-Martin A, Cufi S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, et al. Metformin: multi-faceted protection against cancer. Oncotarget 2011;2:896–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pierotti MA, Berrino F, Gariboldi M, Melani C, Mogavero A, Negri T, et al. Targeting metabolism for cancer treatment and prevention: metformin, an old drug with multi-faceted effects. Oncogene 2013;32:1475–87. [DOI] [PubMed] [Google Scholar]
- [15].He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol: Official J Eur Soc Med Oncol/ESMO 2012;23:1771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol: Official J Am Soc Clin Oncol 2013;31:3069–75. [DOI] [PubMed] [Google Scholar]
- [17].Spratt DE, Zhang C, Zumsteg ZS, Pei X, Zhang Z, Zelefsky MJ. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol 2013;63:709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wan G, Yu X, Chen P, Wang X, Pan D, Wang X, et al. Metformin therapy associated with survival benefit in lung cancer patients with diabetes. Oncotarget 2016;7:35437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Du L, Wang M, Kang Y, Li B, Guo M, Cheng Z, et al. Prognostic role of metformin intake in diabetic patients with colorectal cancer: an updated qualitative evidence of cohort studies. Oncotarget 2017;8:26448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guo J, Xu K, An M, Zhao Y. Metformin and endometrial cancer survival: a quantitative synthesis of observational studies. Oncotarget 2017;8:66169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhou DC, Gong H, Tan CQ, Luo JQ. Prognostic significance of anti-diabetic medications in pancreatic cancer: a meta-analysis. Oncotarget 2017;8:62349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rego DF, Pavan LM, Elias ST, De Luca Canto G, Guerra EN. Effects of metformin on head and neck cancer: a systematic review. Oral Oncol 2015;51:416–22. [DOI] [PubMed] [Google Scholar]
- [23].Kwon M, Roh JL, Song J, Lee SW, Kim SB, Choi SH, et al. Effect of metformin on progression of head and neck cancers, occurrence of second primary cancers, and cause-specific survival. Oncologist 2015;20:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sandulache VC, Hamblin JS, Skinner HD, Kubik MW, Myers JN, Zevallos JP. Association between metformin use and improved survival in patients with laryngeal squamous cell carcinoma. Head & Neck 2014;36:1039–43. [DOI] [PubMed] [Google Scholar]
- [25].Spratt DE, Beadle BM, Zumsteg ZS, Rivera A, Skinner HD, Osborne JR, et al. The influence of diabetes mellitus and metformin on distant metastases in oropharyngeal cancer: a multicenter study. Int J Rad Oncol Biol Phys 2016;94:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. SEER-Medicare Linked Database.
- [27]. CMS Program History.
- [28].Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. New Engl J Med 2012;367:1792–802. [DOI] [PubMed] [Google Scholar]
- [29]. Chronic Conditions Data Warehouse.
- [30].Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiology 2000;53:1258–67. [DOI] [PubMed] [Google Scholar]
- [31].Graubard BI, Korn EL. Predictive margins with survey data. Biometrics 1999;55:652–9. [DOI] [PubMed] [Google Scholar]
- [32].Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol 2014;43:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kleinbaum DGKM. Evaluating the proportional hazards assumption, survival analysis Springer; 2012. p. 161–200. [Google Scholar]
- [34].Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Investigation 2001;108:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab 2013;17:113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007;12:9–22. [DOI] [PubMed] [Google Scholar]
- [37].Chang PH, Yeh KY, Wang CH, Chen EY, Yang SW, Chou WC, et al. Impact of metformin on patients with advanced head and neck cancer undergoing concurrent chemoradiotherapy. Head & Neck 2017;39:1573–7. [DOI] [PubMed] [Google Scholar]
- [38].Bailey CJ. Hypoglycaemic, antihyperglycaemic and antidiabetic drugs. Diabetic Med: J Br Diabetic Assoc 1992;9:482–3. [DOI] [PubMed] [Google Scholar]
- [39].Bailey CJ. Biguanides and NIDDM. Diabetes Care 1992;15:755–72. [DOI] [PubMed] [Google Scholar]
- [40].Bailey CJ, Turner RC. Metformin. New Engl J Med 1996;334:574–9. [DOI] [PubMed] [Google Scholar]
- [41].Gupta SC, Sung B, Prasad S, Webb LJ, Aggarwal BB. Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol Sci 2013;34:508–17. [DOI] [PubMed] [Google Scholar]
- [42].Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res: Official J Am Assoc Cancer Res 2003;9:10–9. [PubMed] [Google Scholar]
- [43].Pavan LM, Rego DF, Elias ST, De Luca Canto G, Guerra EN. In vitro anti-tumor effects of statins on head and neck squamous cell carcinoma: a systematic review. PloS One 2015;10:e0130476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. New Engl J Med 2009;360:626–33. [DOI] [PubMed] [Google Scholar]
- [45].Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer drugs in the United States: Justum Pretium–the just price. J Clin Oncol: Official J Am Soc Clin Oncol 2013;31:3600–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mailankody S, Prasad V. Five years of cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol 2015;1:539–40. [DOI] [PubMed] [Google Scholar]
- [47].Tsakiridis T, Hu C, Skinner H, Santana-Devila R, Lu B, Erasmus J, et al. NRG-LU001: a phase II trial investigating metformin as a chemo-radio-sensitizer in locally advanced non-small cell lung cancer (NSCLC). J Thoracic Oncol 2016;11:S49–50. [Google Scholar]