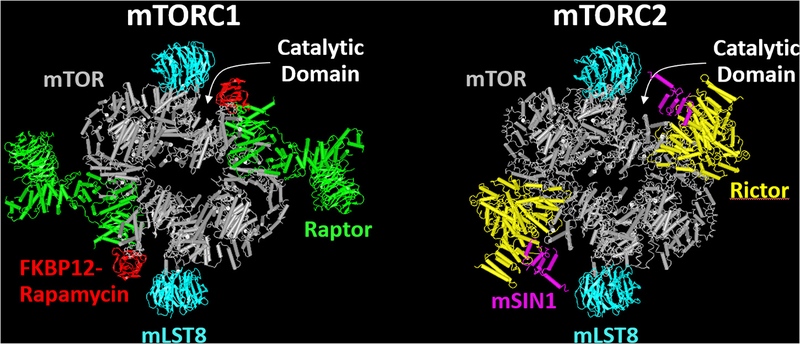
Figure 1.
The web-based version of iCn3D was used to visualize and compare the structures of mechanistic target of rapamycin complex 1 (mTORC1) and mTORC2 that were reported by Aylett et al., 2016 (17) and Chen et al., 2018 (20), respectively. Both mTORC1 and mTORC2 are composed of two mTOR (gray) and two mLST8 (blue) molecules. The defining feature of mTORC1 is the presence of two raptor molecules (green) which deliver mTORC1-specific substrates to the catalytic domain of mTOR. The FKBP12-rapamycin complexes (red) bind to mTOR in the region that lies between raptor and mTOR’s catalytic domain, blocking access of some, but not all, of mTORC1’s substrates to the catalytic domain. The defining feature of mTORC2 is the presence of two rictor (yellow) and two mSIN1 (purple) molecules which sterically inhibit the ability of FKBP12-rapamycin complexes to bind to mTOR.