ABSTRACT
Background
We have observed in the clinic that a number of patients with Parkinson's disease (PD) can suppress their tremor at will for brief periods, by conscious mental processes. To our knowledge, the ability to consciously diminish one's resting tremor has not yet been reported nor assessed quantitatively.
Objective
To provide the first detailed systematic investigation of the phenomenon of voluntary tremor suppression in PD.
Methods
We examined changes in tremor characteristics during voluntary tremor suppression in 37 PD patients (on medication) presenting with rest tremor in their upper limb. We measured tremor oscillations with a triaxis accelerometer on the index finger of the most‐affected hand (n = 27). With surface electromyography (EMG), we measured changes in neuromuscular activity of the forearm flexor digitorum superficialis and extensor digitorum muscles (n = 15). Participants completed four 1‐minute trials, consisting of alternating consecutive 30‐second periods of resting tremor and 30‐second periods of attempted tremor suppression.
Results
Bayesian multilevel modeling revealed that attempted voluntary tremor suppression did indeed reduce tremor amplitude (peak power) of the acceleration signal and increased tremor frequency of the acceleration and EMG signals. Relative EMG power in the 3‐ to 8‐Hz tremor band was also smaller. Tremor suppression was not by enhanced voluntary contraction of the relevant muscle pairs.
Conclusions
We present novel empirical evidence that PD resting tremor can be suppressed by an act of will, as evidenced by significant modulation of key neurophysiological tremor characteristics. These data highlight that it is possible to exert significant conscious control over parkinsonian resting tremor.
Keywords: electromyography, Parkinson's disease, tremor, volition, voluntary suppression
Tremor is one of the cardinal signs that characterizes Parkinson's disease (PD).1 Although not universally present, it is the most common symptom reported at the onset of the disease.2 Resting tremor comprises rhythmical and involuntary oscillations, typically at a frequency between 3 to 6 Hz, in a body part that is not voluntarily activated.3 It can be debilitating and contribute to a decreased quality of life and can severely disrupt activities of daily living and lead to social isolation.2 The neurophysiological mechanisms underlying resting tremor are complex and remain poorly understood. Treatment for tremor has proven to be difficult given that dopaminergic medication is not reliably effective,4 as it is for other symptoms of PD. Surgical interventions, such as ablation or DBS, have established efficacy in reducing parkinsonian tremor,5 but surgery is typically limited to severe cases.
We have encountered anecdotal evidence from a number of patients that they have adopted a coping strategy to manage their tremor through voluntary suppression. They report suppressing their tremor, to some degree and for brief periods, purely by an act of will, without the use of external aids, but rather by conscious mental processes. Despite an abundance of research on parkinsonian resting tremor focusing on its description and pathophysiology, to our knowledge, the ability to exert conscious control to diminish it has not been reported nor assessed empirically. We therefore sought to provide the first detailed description of changes in the neurophysiological characteristics of resting hand tremor associated with voluntary tremor suppression in patients with idiopathic PD. We used accelerometry and electromyography (EMG) to assess the amplitude, power, and frequency of tremor oscillations.
Patients and Methods
Participants
One hundred twenty people with PD, known to have resting tremor in their upper and/or lower limb(s) from previous International Parkinson and Movement Disorder Society (MDS)‐UPDRS assessments, on our New Zealand Brain Research Institute research volunteer database, were sent a questionnaire asking them to respond to questions about having resting tremor and their self‐perceived ability to temporarily suppress it by an act of will. Of 91 responses, 7 people indicated that they had tremor in their leg(s) only and 16 people indicated they only had observable resting tremor when off their regular medication (see inclusion criteria below), leaving 68 people who were contacted and invited to participate in the study. Of these 68, 31 people declined; thus, 37 participants, aged 51 to 80 years (71 ± 6 years; 10 female), were recruited and participated in this study, including those both with (n = 24) and without (n = 13) self‐reported tremor suppression ability. Participants were included if they: (1) met the UK Brain Bank criteria for idiopathic PD6; (2) had observable resting tremor in their upper limb(s)3 when on their regular medication; and (iii) did not meet Level I criteria for dementia.7 Participants remained on medication throughout the study. Exclusion criteria included atypical parkinsonian disorder, history of other neurological conditions, and major psychiatric disorder in the previous 12 months (as assessed by medical history). All participants provided written informed consent and received monetary compensation of travel costs. The study was approved by the Southern Health and Disability Ethics Committee of the New Zealand Ministry of Health.
Tremor Testing
Tremor was assessed in each participant's upper limb most affected by rest tremor, as determined by patient subjective reports, and corroborated by the MDS‐UPDRS Part III motor item 3.17,8 performed at the end of the experimental session. Participants were seated in front of a computer screen, with their forearm and wrist supported by a table and their hand dangling unsupported over the edge of the table.5, 9 We acquired tremor oscillations using a calibrated triaxial accelerometer positioned over the middle phalanx of the index finger. Surface EMG was recorded from the flexor digitorum superficialis (FDS) and extensor digitorum communis (EDC) muscles of the most‐affected arm (see Supporting Information).
Tremor was measured under two instruction conditions: During resting tremor periods, participants viewed the word “REST” on the computer screen for 30 seconds and were instructed to relax their forearm and hand muscles, to allow their wrist and hand to hang freely, and to let their tremor occur naturally without any form of suppression. During tremor suppression, participants viewed the word “SUPPRESS” for 30 seconds and were instructed to attempt to suppress their tremor during this period, using whatever strategy they felt would work best for them, even if they found it difficult to do so. Participants completed four trials of each instruction, continuously alternating between 30 seconds of resting tremor and 30 seconds of tremor suppression. At the end of the experiment, participants were debriefed and asked about any strategies utilized and their self‐perceived ability to suppress their tremor (both during the experimental trials and outside of the laboratory environment; see Supporting Information).
Statistical Analyses
For each trial and for each instruction, we obtained the peak power and frequency of peak power from the acceleration and EMG signals, as well as the amplitude of muscle activity and relative EMG power (see Supporting Information). Trials with absent or minimal tremor or with minimal or noisy EMG activity during the Rest instruction were excluded from statistical analyses (see Supporting Information for details on tremor identification and data exclusion). Given that the participant's upper limbs were affected differently by tremor (e.g., some showed prominent tremor in the fingers, whereas others exhibited pill‐rolling tremor with predominant thumb and wrist involvement), we chose to focus our analyses of muscle activity on data from a subset of patients whose tremor involved flexion and extension of the index finger and hence robust activation of the FDS and EDC muscles.
Changes in tremor characteristics between the resting tremor and tremor suppression instructions were examined using Bayesian multilevel modeling. The probabilistic language Stan 10 was used along with the R packages rstan (v2.16.2) and brms (v1.10.0) to fit the Bayesian model and generate all estimates within the R statistical environment (v3.3.111). The models included Instruction (rest, suppress) as the primary predictor and allowed the intercept to vary by Participant, and by Instruction within Participant. Data for peak power were log transformed before analysis. Results are reported as the means of the posterior distribution, together with 95% credible intervals. Results for EDC are illustrated in the Supporting Information.
Results
Characterising Tremor During Rest and Suppression in Individual Participants
Participants reported using a range of strategies to suppress their tremor, including visualization; relaying that they “think about the tremor stopping and concentrate” or “maintain focus on my arm,” and relaxation; “I relax my muscles to the best of my ability, imaging that my arm is heavy.” Two participants used controlled breathing to help suppress their tremor involving “inhaling a long breath, and exhaling slowly,” and 1 participant described their suppression strategy as “releasing my tremor” through meditation practice. Participants reported they were more successful suppressing their tremor when they were more relaxed or calm, and “not under stress.” Of the 24 participants tested who self‐reported they could volitionally suppress their tremor, half felt they could “substantially” reduce their tremor “most of the times” that they tried; the other half reported a “moderate reduction.” Two‐thirds of these 24 participants reported that they could suppress tremor for longer than 1 minute, whereas those who suppressed for shorter durations (10–60 seconds) indicated that they could do this “every time I try” and that they could achieve “sustained suppression.” Importantly, two‐thirds reported they could suppress “on command” as well as “while doing another task” (e.g., talking to someone, watching television). During the experimental testing, however, the ability to suppress tremor was not necessarily reliably present in all of those who self‐reported the ability. Conversely, those who reported that they could not suppress, in fact, often could after actually attempting to do so.
Of the 37 participants tested, 10 did not reliably exhibit tremor that was distinguishable from background noise during the accelerometry recordings of the resting tremor trials, despite having a score ≥1 when assessed on MDS‐UPDRS items 3.17 and 3.18. Thus, data for 27 participants were retained for the primary analyses of the accelerometry data (see Table 1). Of these 27 participants, a subgroup of 15 participants had tremor primarily involving (index) finger flexion and extension; thus, preliminary secondary analyses on FDS and EDC muscle activity data were conducted only in this subgroup (together with their corresponding accelerometry data).
Table 1.
Demographics and clinical characteristics of PD participants included in the primary analyses of the accelerometry data and the secondary analyses that included examination of muscle activity
| Primary Analyses n = 27 | Secondary Analyses n = 15 | |
|---|---|---|
| Age (years) | 70 (7) [51‐80] | 70 (7) [51‐78] |
| Sex (male:female) | 18:9 | 12:3 |
| Handedness (left:right) | 2:25 | 2:13 |
| Most‐affected side (left:right) | 14:13 | 10:5 |
| Duration of disease (years) | 12 (7) [1–26] | 11 (7) [1–24] |
| H & Y30 , a | 2.3 (0.7) [1–4] | 2.3 (0.6) [1–4] |
| MDS‐UPDRS Part III motora | ||
| Total score | 33 (14) [6–65] | 33 (15) [7–65] |
| Rest tremorb (most‐affected hand) | 1.9 (0.9) [1–4] | 2.3 (0.8) [1–4] |
| Rest tremorb (least‐affected hand) | 0.7 (1.0) [0–3] | 0.7 (1.1) [0–3] |
| Constancy of rest tremorc | 2.4 (1.2) [1–4] | 2.9 (1.1) [1–4] |
| Time since medication (hours) | 3.3 (1.3) [1.00–6.25] | 3.5 (1.5) [1.75–6.25] |
Figure 1A,B illustrates single‐trial acceleration time series and their corresponding acceleration power spectra from 2 participants. Although participant 02 (Fig. 1A) had smaller tremor magnitude than participant 33 during the Rest instruction (Fig. 1B), both participants were able to reduce their tremor, with varying success, over the four trials. Both participants demonstrated an ability to suppress their tremor within 1 second of the onset of the Suppress instruction cue, and it is notable that tremor sometimes remained suppressed at the beginning of the following resting tremor trial (i.e., hysteresis effect), despite the participants reporting that they were no longer actively trying to suppress (e.g., Fig. 1A, beginning of trials 2–4). The power spectra show large reductions in peak power between 3 and 6 Hz, as well as obvious rightward shifts in the peak power frequency during suppression.
Figure 1.
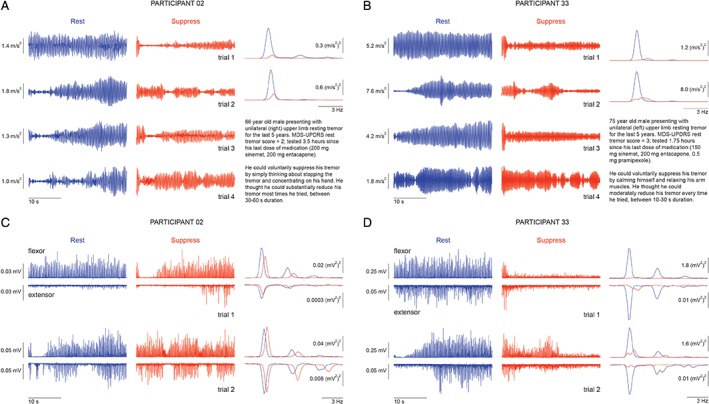
Example accelerometry and EMG recordings, together with brief clinical descriptions, from 2 PD participants (participant 02 [A,C] and participant 33 [B,D]) who could voluntarily suppress their resting tremor. (A,B) Accelerometry data during resting tremor (left traces within each panel) and voluntary tremor suppression (middle traces) for all four trials and the corresponding power spectra under rest (blue) and suppress (orange) instructions (right traces). (C,D) Flexor and extensor EMG recordings during resting tremor (left traces within each panel) and voluntary tremor suppression (middle traces) for the first two trials (related to the first two trials in A,B) and the corresponding power spectra under rest (blue) and suppress (orange) instructions (right panels). See also Supporting Information Figure S1 for representative data with an expanded time scale and unrectified EMG signal.
Primary Analyses: Accelerometry‐Derived Tremor Amplitude (Peak Power) and Dominant Frequency
Figure 2 illustrates the group results (n = 27), highlighting the influence of voluntary suppression on key tremor characteristics derived from accelerometry. Consistent with the observations shown at the individual level in Figure 1, Bayesian multilevel modeling at the group level estimated that tremor amplitude (peak power) was reduced by 0.7 log((m/s2)2/Hz), 95% uncertainty interval [0.4, 1.0], during the Suppress instruction compared to the Rest instruction (Fig. 2A), and the frequency of peak power was increased by 0.4 Hz [0.2, 0.5] during Suppress compared to Rest (Fig. 2B).
Figure 2.
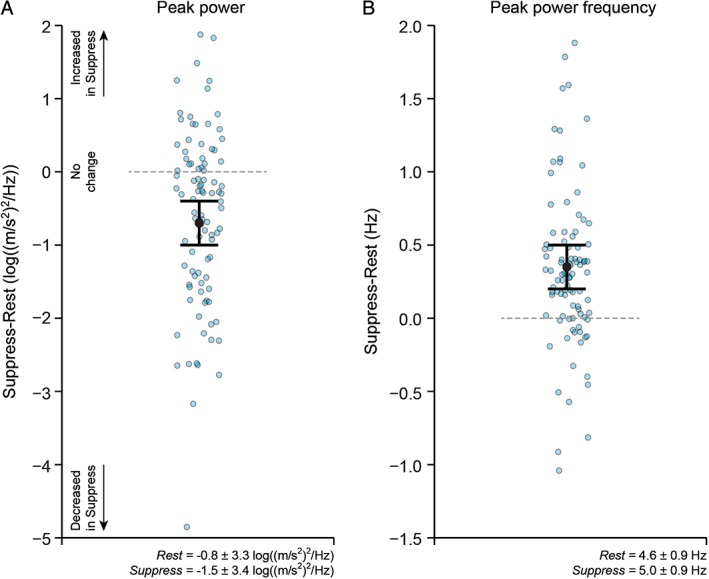
Changes in accelerometry‐derived tremor characteristics (primary analyses; n = 27) during resting tremor compared to during voluntary tremor suppression in Parkinson's disease. Suppression of tremor was associated with a decrease in peak power (A) and an increase in the frequency of peak power (B). Graphs plot the estimate for the mean population change (Suppress minus Rest), together with 95% uncertainty intervals. Data for individual trials (Suppress minus Rest) are also shown (blue circles). Data for peak power were log transformed before analysis. The horizontal line at value = 0 represents no change in the measure of interest between the Rest and Suppress instructions; data values below 0 indicate that the measure of interest was smaller in the Suppress compared to Rest instruction; data values above 0 indicate that the measure of interest was greater in the Suppress compared to Rest instruction. The group mean ± standard deviation for the Rest and Suppress instructions are included as text inserts.
Secondary Analyses: Accelerometry With Concomitant EMG Data
Analyses of the accelerometry data in the subgroup of 15 participants, whose tremor involved prominent movement of the index finger and thus FDS and EDC activation, revealed the same pattern of results as the full group (of 27 participants). Compared to the Rest instruction, tremor during the Suppress instruction was associated with a decrease in peak power of 0.9 log((m/s2)2/Hz) [0.5, 1.4] and an increase in the peak power frequency of 0.4 Hz [0.3, 0.6], from 4.3 to 4.7 Hz.
Figure 1C,D illustrates exemplar single‐trial flexor and extensor EMG activity and the corresponding power spectra for trials 1 and 2 from the same participants and trials, as shown in Figure 1A,B (see also Supporting Information Fig. S1). Overall, in the group of 15 participants, mean FDS and EDC muscle activity were similar during the Rest and Suppress instructions (Fig. 3A and Supporting Information Fig. S2A). Peak power in the EMG spectra was reduced during the Suppress compared to the Rest instruction in FDS (by 0.5 log((mV)2/Hz) [0.1, 0.9]; Fig. 3B), but not in EDC (Supporting Information Fig. S2B). However, the frequency of peak power during the Suppress instruction increased in both FDS (by 0.5 Hz [0.2, 0.8]; Fig. 3C) and EDC (by 0.4 Hz [0.2, 0.6]; Supporting Information Fig. S2C). As expected with resting tremor, the majority of FDS and EDC EMG power (~65%) was contained in the 3‐ to 8‐Hz tremor band during resting tremor and tremor suppression (Fig. 3D and Supporting Information Fig. S2D). The relative power in the tremor band was reduced during the Suppress compared to Rest instruction (by 0.08 [0.05 0.12] for FDS, by 0.07 [0.04, 0.10] for EDC), but was similar between Rest and Suppress in the 1‐ to 3‐Hz low‐frequency band.
Figure 3.
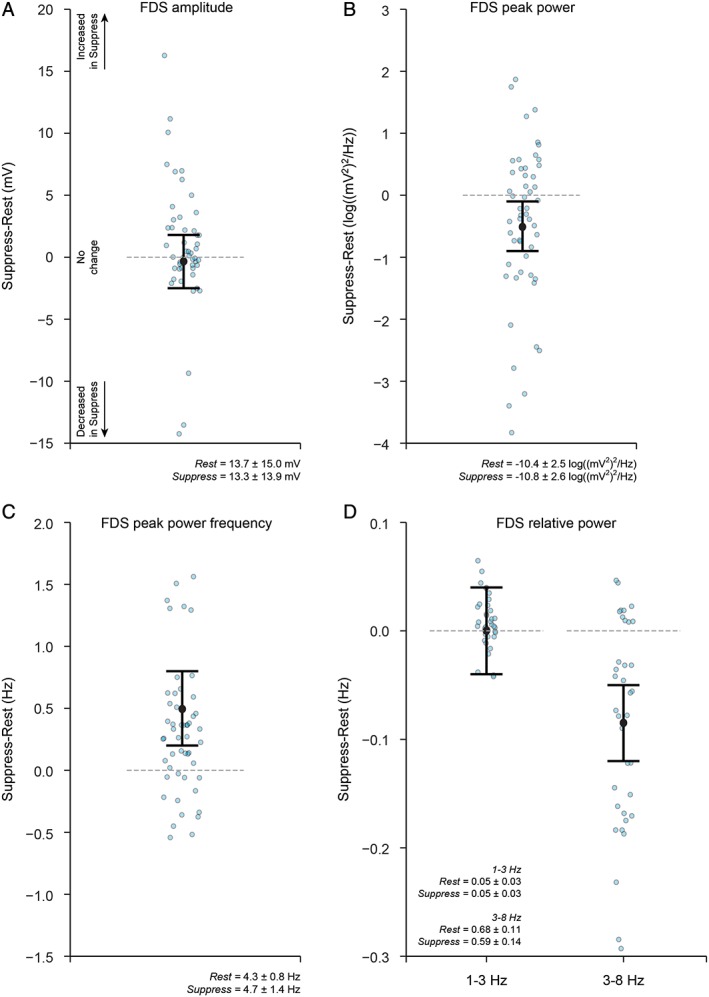
Changes in EMG‐derived tremor characteristics (secondary analyses; n = 15) during resting tremor compared to during voluntary tremor suppression in PD. (A) Forearm flexor muscle FDS amplitude, (B) FDS peak power, (C) FDS peak power frequency, and (D) FDS relative power in the 1‐ to 3‐Hz and 3‐ to 8‐Hz bands. FDS amplitude remained similar between the Rest and Suppress instructions; however, FDS peak power was smaller, and the peak frequency of FDS oscillation increased during suppression of tremor. The majority of power was contained in the 3‐to 8‐Hz tremor band. Relative power in the tremor band was smaller during suppression of tremor, but similar between the Rest and Suppress instructions in the 1‐ to 3‐Hz low‐frequency band. Graphs plot the estimate for the mean population change (Suppress minus Rest), together with 95% uncertainty intervals. Data for individual trials (Suppress minus Rest) are also shown (blue circles). Data for peak power were log transformed before analysis. The horizontal line at value = 0 represents no change in the measure of interest between the Rest and Suppress instructions; data values below 0 indicate that the measure of interest was smaller in the Suppress compared to Rest instruction; data values above 0 indicate that the measure of interest was greater in the Suppress compared to Rest instruction. The group mean ± standard deviation for the Rest and Suppress instructions are included as text inserts.
Insights From EMG Into Potential Suppression Strategies
Given that several participants described using muscular relaxation (e.g., participant 33; Fig. 1D) and visualization techniques to suppress their tremor, further examination of the EMG data could possibly reveal insights into potential strategies to voluntarily diminish tremor involving modulation of neuromuscular activity; such strategies may be masked when averaging across trials and/or group (c.f., Fig. 3A and Supporting Information Fig. S1A). We therefore conducted additional exploratory analyses, where we classified and then defined each trial as either “Reduced EMG” or “Non‐reduced EMG” trials, according to whether the magnitude of EMG activity was reduced during tremor suppression compared to resting tremor (see Supporting Information).
These exploratory analyses showed that a reduction (suppression) of forearm muscle activity was evident in 54% of trials. Reduced peak power of tremor acceleration during the Suppress compared to Rest instruction was found irrespective of whether the magnitude of EMG activity remained the same (Non‐reduced EMG trials) or decreased (Reduced EMG trials; Fig. 4A). A larger reduction in peak power of acceleration (of 1.3 log((m/s2)2/Hz) [0.5, 2.1]) was, however, achieved during tremor suppression trials with Reduced EMG (muscular relaxation) compared to Non‐reduced EMG trials (Fig. 4A).
Figure 4.
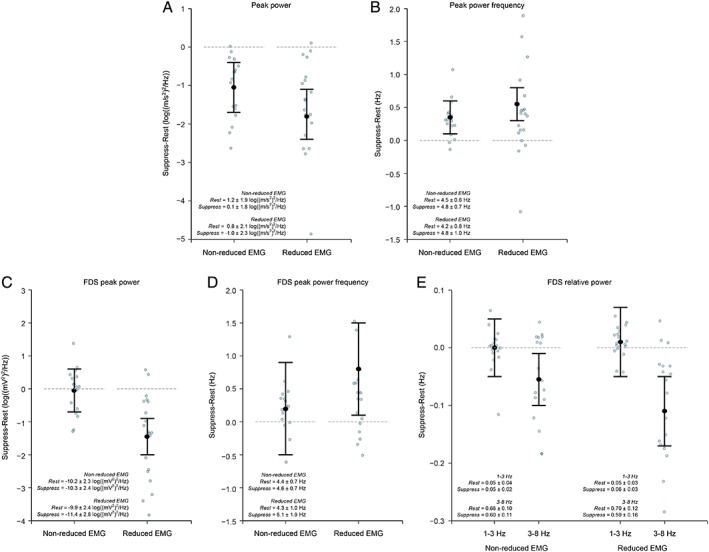
Exploratory analyses of EMG data (n = 15) during resting tremor compared to during voluntary tremor suppression provide insight into strategies to voluntarily diminish resting tremor in PD. Trials were categorised as nonreduced or reduced EMG according to whether mean EMG activity was reduced during the Suppress compared to the Rest instruction (see text and Supporting Information for details). (A) Acceleration peak power, (B) acceleration peak power frequency, (C) FDS peak power, (D) FDS peak power frequency, and (E) FDS relative power. Suppression of tremor involving reduced EMG amplitude resulted in greater reductions in peak power and relative power in the 3‐ to 8‐Hz tremor band, as well as increased frequency of tremor oscillations. Graphs plot the estimate for the mean population change (Suppress minus Rest), together with 95% uncertainty intervals. Data for individual trials (Suppress minus Rest) are also shown (blue circles). Data for peak power were log transformed before analysis. The horizontal line at value = 0 represents no change in the measure of interest between the Rest and Suppress instructions; data values below 0 indicate that the measure of interest was smaller in the Suppress compared to Rest instruction; data values above 0 indicate that the measure of interest was greater in the Suppress compared to Rest instruction. The group mean ± standard deviation for the Rest and Suppress instructions are included as text inserts.
Tremor suppression by Reduced EMG also resulted in smaller FDS peak power by 1.4 log((mV)2/Hz) [0.9, 2.0] and EDC peak power by 0.9 log((mV)2/Hz) [0.3, 1.4], whereas muscle peak power remained similar from Rest to Suppress for the Non‐reduced EMG trials (FDS, –0.1 log((mV)2/Hz [–0.7, 0.6]; EDC, 0.2 log((mV)2/Hz [–0.4, 0.8]; Fig. 4C and Supporting Information Fig. S3A). Similarly, the dominant frequency of EMG oscillation increased during the Suppress compared to Rest instruction for Reduced EMG trials, but not for the Non‐reduced EMG trials (Fig. 4D and Supporting Information Fig. S3B). Relative power in FDS was reduced in the 3‐ to 8‐Hz tremor band during the Suppress compared to Rest instruction for Reduced EMG (by 0.11 [0.05, 0.17]) and Non‐reduced EMG (by 0.05 [0.01, 0.10]) trials (Fig. 4E). EMG relative power was similar from Rest to Suppress in the low‐frequency 1‐ to 3‐Hz band (Fig. 4E and Supporting Information Fig. S3C).
Discussion
We examined changes in the neurophysiological characteristics of resting hand tremor during attempted voluntary tremor suppression in PD. Using accelerometry and EMG, we found novel support for patient anecdotes and our clinical observations that parkinsonian resting tremor can be modulated voluntarily, without external aids. During voluntary tremor suppression periods, tremor amplitude (peak power) was reduced whereas tremor frequency increased. EMG gave some insight into the mechanisms at a muscular level: Suppression of tremor observed in accelerometry recordings was found regardless of whether the magnitude of forearm EMG activity was modulated or not. This suppression was associated with a decrease in relative power in the 3‐ to 8‐Hz tremor band, but without any accompanying change in relative power in the 1‐ to 3‐Hz low‐frequency band related to tonic muscle activity.
Besides pharmacotherapy and neurosurgical options for managing PD tremor, laboratory approaches have focused on attenuating tremor amplitude through external aids, such as augmenting muscle contraction through mechanical loading,12 neurostimulation to the limb by neuroprostheses,13 or rhythmic transcranial current stimulation.14 Here, we show that attenuation of tremor in PD patients on their regular antiparkinsonian medication can also be achieved voluntarily, by a conscious act of will alone. Neither augmented feedback nor the application of external loads or devices to the limb are mandatory to reduce tremor amplitude.
Given that rest tremor typically diminishes or becomes absent during voluntary movement,15 it was possible that the observed changes in accelerometry‐derived tremor characteristics reflected voluntary activation of muscle. Thus, we assessed the magnitude and relative power of concomitant neuromuscular activity to examine whether voluntary tremor suppression was underpinned by increased tonic activity of the forearm muscle(s). This was not the case—in participants showing prominent involvement of FDS and EDC in the generation of resting tremor (secondary analysis), we found no change in mean EMG activity or EMG relative power in the 1‐ to 3‐Hz band during tremor suppression compared to tremor at rest. These observations indicate that it is unlikely that participants adopted a strategy of (volitionally) increasing muscle tension as a means to stabilize the effector and hence to diminish tremor. This is consistent with a study demonstrating voluntary modulation of physiological tremor,16 where explicit instructions to use muscular co‐contraction to stabilize the finger amplified, rather than reduced, tremor amplitude.
A range of suppression strategies were reported by our participants, with a common underlying theme that participants thought they were more successful when “relaxation” of the affected limb and muscles was applied, such as through visualization, controlled breathing, or concentration, and in the absence of stress. Evidence from the EMG data supports this notion: Volitional strategies that resulted in relaxation at the muscular level (Reduced EMG trials) had greater influence on EMG peak power and peak power frequency, and the relative EMG power in the 3‐ to 8‐Hz tremor band compared to Non‐reduced EMG trials, resulting in more effective tremor suppression. Moreover, whereas peak power and 3‐ to 8‐Hz relative power in FDS and EDC was reduced during relaxation, no change in muscular peak power was observed during suppression in the Non‐reduced EMG trials, and there was also no change in 1‐ to 3‐Hz relative power during suppression (in Reduced and Non‐reduced EMG trials). These results provide further evidence that a strategy of enhanced muscular contraction to diminish tremor was not utilized by the participants. One previous study examining relaxation techniques demonstrated reduced parkinsonian tremor associated with externally scripted relaxation‐guided imagery that had no specific reference to tremor, but, in contrast to our results, not with self‐relaxation.17 Nonetheless, we extend these findings and other previous studies that have highlighted the therapeutic role of relaxation in ameliorating PD motor symptoms,17, 18, 19, 20 which, conversely, stress exacerbates. Clinically, this points to the possibility of a patient‐driven therapeutic approach focusing on relaxation techniques as a viable noninvasive strategy for minimizing tremor impact. The addition of heart rate or pupil diameter recordings to the current protocol may also offer independent insight into relaxation or other strategies adopted to voluntarily suppress tremor. Whether the presence of stressors interferes with the ability to voluntarily suppress tremor and exacerbates EMG amplitude and power is the focus of our current research.
Interestingly, voluntary tremor suppression increased the peak frequency of tremor oscillations. Increased parkinsonian tremor frequency has been found during stress,21 in on versus off antiparkinsonian medication states,5, 22 and with DBS of the STN.5, 22 Tremor frequency as determined from accelerometry includes contributions from both central oscillators and peripheral mechanical‐reflex oscillators (which, under natural conditions, have a higher frequency than central oscillators),23 whereas EMG frequency shifts primarily reflect modulation of the central oscillator(s). The increases in tremor frequency apparent in both accelerometry and EMG recordings thus likely indicate effects upon pathological central oscillator(s). The mechanisms underlying increased tremor frequency and reduced magnitude during tremor suppression (and, more generally, the genesis of tremor) are unclear. However, it is possible that voluntary suppression dampened the pathological central oscillator(s), rather than directly changing its frequency, allowing other oscillators (e.g., mechanical‐reflex) to play a more dominant role.5, 23
There is converging evidence that resting tremor is linked to altered activity in both the basal ganglia‐cortical and cerebello‐thalamo‐cortical circuits and that both circuits converge in the motor cortex.24 In particular, it is thought the cerebello‐thalamo‐cortical circuit controls tremor magnitude, whereas the striato‐pallidal circuit triggers tremor episodes.24, 25 Thus, presumed higher‐order top‐down processes associated with the volitional suppression of tremor, through relaxation or otherwise, may interact with resting tremor processes in one or both of these circuits. That involuntary tremor may be susceptible to “voluntary override” or inhibitory control is evident clinically: Tremor diminishes with ipsilateral voluntary limb movement, yet increases with contralateral movement,4 and there is ample evidence that PD tremor and repetitive voluntary movements share common cortical networks.26, 27, 28 Moreover, the notion that involuntary movements can be consciously suppressed is consistent with the view of tic control through voluntary inhibitory motor processes (e.g., in Tourette syndrome).29 We did not directly address how the tremor circuits and other brain regions involved in integrating volition and motor control might be modulated by voluntary tremor suppression, but we intend to follow this up with functional neuroimaging studies. Increased understanding of the neural mechanisms underpinning this phenomenon could offer novel insights into tremor pathophysiology and may allow targeting these brain regions for future management of parkinsonian tremor, either by exogenous (e.g., DBS, transcranial magnetic stimulation) or endogenous (e.g., cognitive behavioral or biofeedback) activation/deactivation. In conclusion, our data show that parkinsonian resting tremor can be suppressed by an act of will using conscious mental processes, highlighting that tremor can, at least partially, be voluntarily modulated.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
R.L.B.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
M.R.M.: 1A, 1B, 2C, 3B
T.J.A.: 1A, 1B, 2C, 3B
D.J.M.: 2A, 2C, 3B
Disclosures
Ethical Compliance Statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. All participants provided written informed consent and received monetary compensation of travel costs. The study was approved by the Southern Health and Disability Ethics Committee of the New Zealand Ministry of Health.
Funding Sources and Conflicts of Interest
This work was supported by a Neurological Foundation of New Zealand Project Grant (1540‐PG to R.L.B.) and the New Zealand Brain Research Institute. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the previous 12 months
R.L.B. received funding from the Neurological Foundation of New Zealand and the New Zealand Brain Research Institute. D.J.M. received funding from the Canterbury Medical Research Foundation, the Neurological Foundation of New Zealand, and the New Zealand Brain Research Institute. M.R.M. received funding from the New Zealand Brain Research Institute and the Neurological Foundation of New Zealand. T.J.A. received funding from the Canterbury District Health Board and the University of Otago.
Supporting information
Figure S1. Example accelerometry and EMG recordings from 2 PD participants (participant 02 [A,C] and participant 33 [B,D]) who could voluntarily suppress their resting tremor.
Figure S2. Changes in EDC EMG‐derived tremor characteristics (secondary analyses; n = 15) during resting tremor compared to during voluntary tremor suppression in PD.
Figure S3. Exploratory analyses of EDC EMG data (n = 15) during resting tremor compared to during voluntary tremor suppression provide insight into strategies to voluntarily diminish resting tremor in PD.
Appendix S1. Supporting information.
Acknowledgments
We thank Leslie Livingston for assistance with participant recruitment, as well as all the research volunteers and their families for generously giving their time to participate in this research.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci 2003;991:1–14. [DOI] [PubMed] [Google Scholar]
- 2. Fishman PS. Paradoxical aspects of Parkinsonian tremor. Mov Disord 2008;23:168–173. [DOI] [PubMed] [Google Scholar]
- 3. Bhatia KP, Bain P, Bajaj N, et al. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 2018;33:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duval C, Daneault JF, Hutchison WD, Sadikot AF. A brain network model explaining tremor in Parkinson's disease. Neurobiol Dis 2016;85:49–59. [DOI] [PubMed] [Google Scholar]
- 5. Sturman MM, Vaillancourt DE, Verhagen Metman L, Bakay RAE, Corcos DM. Effects of subthalamic nucleus stimulation and medication on resting and postural tremor in Parkinson's disease. Brain 2004;127:2131–2143. [DOI] [PubMed] [Google Scholar]
- 6. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22:1689–1707. [DOI] [PubMed] [Google Scholar]
- 8. Goetz CG, Fahn S, Martinez‐Martin P, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22:41–47. [DOI] [PubMed] [Google Scholar]
- 9. Henderson JM, Yiannikas C, Morris JGL, Einstein R, Jackson D, Byth K. Postural tremor of Parkinson's disease. Clin Neuropharmacol 1994;17:277–285. [DOI] [PubMed] [Google Scholar]
- 10. Carpenter B, Gelman A, Hoffman MD, et al. Stan: a probabilistic programming language. J Stat Softw 2017;76:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 12. Manto M, Topping M, Soede M, et al. Dynamically responsive intervention for tremor suppression. IEEE Eng Med Biol Mag 2003;22:120–132. [DOI] [PubMed] [Google Scholar]
- 13. Gallego JA, Rocon E, Belda‐Lois JM, Pons JL. A neuroprosthesis for tremor mangement through the control of muscle co‐contraction. J Neuroeng Rehabil 2013;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brittain JS, Probert‐Smith P, Aziz TZ, Brown P. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol 2013;23:436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deuschl G, Raethjen J, Baron R, Lindemann M, Wilms H, Krack P. The pathophysiology of parkinsonian tremor: a review. Journal of Neurology 2000;247(Suppl 5):V33–V48. [DOI] [PubMed] [Google Scholar]
- 16. Carignan B, Daneault JF, Duval C. The amplitude of physiological tremor can be voluntarily modulated. Exp Brain Res 2009;194:309–316. [DOI] [PubMed] [Google Scholar]
- 17. Schlesinger I, Benyakov O, Erikh I, Suraiya S, Schiller Y. Parkinson's disease tremor is diminished with relaxtion guided imagery. Mov Disord 2009;14:2059–2062. [DOI] [PubMed] [Google Scholar]
- 18. Raethjen J, Austermann K, Witt K, Zeuner KE, Papengut F, Deuschl G. Provocation of Parkinsonian tremor. Movement Disorders 2008;23:1019–1023. [DOI] [PubMed] [Google Scholar]
- 19. Craig LH, Svircev A, Haber M, Juncos JL. Controlled pilot study of the effects of neuromuscular therapy in patients with Parkinson's disease. Mov Disord 2006;21:2127–2133. [DOI] [PubMed] [Google Scholar]
- 20. Brefel‐Courbon C, Desboeuf K, Thalamas C, Galitzky M, Senard JM, Rascol O, Montastruc JL. Clinical and economic analysis of spa therapy in Parkinson's disease. Movement Disorders 2003;18:578–584. [DOI] [PubMed] [Google Scholar]
- 21. Lee HJ, Lee WW, Kim SK, et al. Tremor frequency characteristics in Parkinson's disease under resting‐state and stress‐state conditions. J Neurol Sci 2016;362:272–277. [DOI] [PubMed] [Google Scholar]
- 22. Sturman MM, Vaillancourt DE, Verhagen Metman L, Sierens K, Bakay RAE, Corcos DM. Deep brain stimulation and medication for Parkinsonian tremor during secondary tasks. Mov Disord 2007;22:1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vaillancourt DE, Sturman MM, Verhagen Metman L, Bakay RAE, Corcos DM. Deep brain stimulation of the VIM thalamic nucleus modifies several features of essential tremor. Neurology 2003;61:919–925. [DOI] [PubMed] [Google Scholar]
- 24. Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain 2012;135:3206–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit in Parkinson's tremor. Ann Neurol 2011;69:269–281. [DOI] [PubMed] [Google Scholar]
- 26. Duffau H, Tzourio N, Caparros‐Lefebvre D, Parker F, Mazoyer B. Tremor and voluntary repetitive movement in Parkinson's disease: comparison before and after L‐dopa with positron emission tomography. Exp Brain Res 1996;107:453–462. [DOI] [PubMed] [Google Scholar]
- 27. Timmermann L, et al. The cerebral oscillatory network of Parkinsonian resting tremor. Brain 2003;126:199–212. [DOI] [PubMed] [Google Scholar]
- 28. Fukuda M, et al. Thalamic stimulation for parkinsonian tremor: correlation between regional cerebral blood flow and physiological tremor characteristics. Neuroimage 2004;21:608–615. [DOI] [PubMed] [Google Scholar]
- 29. Ganos C, Rothwell JC, Haggard P. Voluntary inhibitory motor control over involuntary tic movements. Mov Disord 2018;33:937–‐946. [DOI] [PubMed] [Google Scholar]
- 30. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:424–442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Example accelerometry and EMG recordings from 2 PD participants (participant 02 [A,C] and participant 33 [B,D]) who could voluntarily suppress their resting tremor.
Figure S2. Changes in EDC EMG‐derived tremor characteristics (secondary analyses; n = 15) during resting tremor compared to during voluntary tremor suppression in PD.
Figure S3. Exploratory analyses of EDC EMG data (n = 15) during resting tremor compared to during voluntary tremor suppression provide insight into strategies to voluntarily diminish resting tremor in PD.
Appendix S1. Supporting information.


