Abstract
Aim:
Determine the efficacy of behavioral therapy for urinary symptoms in Parkinson’s disease.
Methods:
Randomized trial of behavioral therapy compared to control condition among adults (aged 54–85 years, 74% male, 10% black/83% white) with Parkinson’s and ≥4 incontinence episodes weekly. Behavioral therapy included pelvic floor muscle exercises, bladder training, fluid and constipation management. Both groups completed bladder diary self-monitoring. Outcomes included diary-derived incontinence and ICIQ-overactive bladder (OAB) score (range 0–16) with bother and quality of life questionnaires (higher scores = worse outcomes).
Results:
53 participants randomized and 47 reported 8-week outcomes including 26 behavioral therapy and 21 control. Behavioral vs. control participants were similar with respect to age (71.0 ± 6.1 vs. 69.7 ± 8.2 years), sex (70% vs. 78% male), motor score, cognition, mean weekly incontinence episodes (13.9 ± 9.6 vs. 15.1 ± 11.1) and OAB symptoms (8.9 ± 2.4 vs. 8.3 ± 2.2). Weekly incontinence reduction was similar between behavioral (−6.2 ± 8.7) and control participants (−6.5 ± 13.8) (p=0.89). After multiple imputation analysis, behavioral therapy participants reported statistically similar reduction in OAB symptoms compared to control ((−3.1 ± 2.8) vs. (−1.9 ± 2.2), p=0.19); however quality of life ((−22.6 ± 19.1) vs. (−7.0 ± 18.4), p=0.048) and bother ((−12.6 ± 17.2) vs. (−6.7 ± 8.8), p=0.037) improved significantly more with behavioral therapy.
Conclusion:
Self-monitoring resulted in fewer urinary symptoms; however, only multicomponent behavioral therapy was associated with reduced bother and improved quality of life. Providers should consider behavioral therapy as initial treatment for urinary symptoms in Parkinson’s disease.
Keywords: Parkinson’s disease, urinary incontinence, overactive bladder, behavioral therapy
Introduction
While Parkinson’s disease is often characterized by motor symptoms (tremor, bradykinesia, rigidity), non-motor symptoms, including urinary symptoms, correlate more closely with impaired well-being as the disease progresses1. Overactive bladder (OAB) symptoms, including urgency, frequency, and nocturia, with or without urgency urinary incontinence, are the most common urinary symptoms of Parkinson’s disease1,2. Because OAB symptoms, such as urgency incontinence and nocturia, are associated with falls3,4 (a cause of increased mortality in Parkinson’s disease5), spouse/caregiver stress6, and, ultimately institutionalization7, it is critical that we provide efficacious therapy for the treatment of urinary symptoms in persons with Parkinson’s disease.
Current guidelines for treatment of urinary symptoms in Parkinson’s disease recommend the use of anticholinergic drugs because they act on muscarinic receptors in the bladder smooth muscle8. There is a large body of evidence supporting the efficacy of anticholinergic drugs for reducing symptoms of OAB in adults; however, only limited data exist for these drugs in Parkinson’s disease. In one pilot study, anticholinergic drug therapy was associated with an improvement in urinary incontinence, but had no impact on urinary frequency9. However, it is well established that people with Parkinson’s’ disease are more susceptible to negative cognitive effects of anticholinergic medications due to their underlying potential to develop cognitive impairment10–12 as well as the most common side effects of constipation and dry mouth13. Therefore, it is important to consider non-pharmacologic approaches to treatment.
Pelvic floor muscle exercise-based behavioral therapy for urinary symptoms requires learning a motor skill and implementing an adaptive behavioral strategy incorporating pelvic floor muscle contraction to delay the need to void when urgency strikes14,15. Most behavioral studies have excluded persons with neurodegenerative disease; however, one study demonstrated the feasibility of pelvic floor muscle exercise-based behavioral therapy to treat urgency incontinence in adults with Parkinson’s disease16. The goal of the present study was to determine the efficacy of behavioral therapy compared to a control condition in a randomized controlled trial.
Methods
We conducted a parallel group randomized clinical trial of pelvic floor muscle exercise-based behavioral therapy compared to a control condition for the treatment of urinary symptoms in Parkinson’s disease. Participants were both Veteran and non-Veteran community-dwelling men and women recruited at two VA medical centers as well as through university affiliates and foundation listings in Atlanta, GA and Birmingham, AL. Recruitment occurred from September 2012 – May 2017. All participants provided written informed consent.
Eligible participants were diagnosed with Parkinson’s disease by a neurologist with additional fellowship training in movement disorders. Participants then had to complete a 7-day bladder diary confirming at least 4 episodes of urinary incontinence. Exclusion criteria included: significant cognitive impairment (as measured by a Montreal Cognitive Assessment17 score of < 18/3018 or the inability to complete an interpretable 7-day bladder diary), significant depression (as measured by a Geriatric Depression Scale19 score of ≥ 10/15), previous intensive pelvic floor muscle therapy, hemoglobin A1c > 8.0%, a post-void residual volume of ≥ 200 mL by bladder ultrasound, use of hemodialysis, poorly controlled heart failure or chronic obstructive pulmonary disease, use of an indwelling catheter, pelvic surgery within the past 12 months, ongoing treatment for cancer related to the lower urinary tract, or life-limiting illness (as determined by the primary investigator or primary care provider). Persons taking medications for lower urinary tract symptoms were eligible if they reported persistent symptoms despite stable dosing for at least 1 month of bladder relaxant and alpha blocker medications, or for at least 6 months for 5-alpha reductase inhibitors. Participants were instructed to maintain a constant dose of these medications during the study. The Movement Disorder Society-Unified Parkinson Disease Rating Scale (MDS-UPDRS) and modified Hoehn & Yahr scale20 were performed during a defined “on” period (when participants perceived their best motor function, usually about 45 minutes after dopaminergic medication).
Based on a previous feasibility study, we estimated a sample size of 50 completed participants (25 in each group) would allow 85% power at a significance level of 5% to detect a difference of 40% between treatment and control groups with regard to weekly urinary incontinence frequency at 8-weeks post-randomization. Study recruitment ended after the recruitment of 53 participants based on the funding award end date.
Computer-generated block randomization codes were generated by the study statistician in blocks of 6 with stratification by site, sex, and weekly incontinence frequency (≤ or > 10 episodes of urinary incontinence per week) to ensure balance between the groups. Individual randomization assignments were stored in sealed, sequentially numbered envelopes by a research staff member who was not directly involved in any other study procedures. After the initial visit to perform baseline assessments, eligible participants were randomly assigned to an intervention group at the second visit by the interventionist unsealing the next available envelope according to the stratification variables.
Control Group
Participants randomized to the control group were asked to maintain 7-day bladder diaries throughout the 8-week study period. After 30 participants were randomized, a blinded interim analysis showed no difference between the groups with regard to change in urinary incontinence frequency ((p=0.9) both groups improved significantly). Therefore, bladder-diary completion was reduced in the control group to only at baseline and 8-weeks post-randomization in order to limit the impact of self-monitoring. In the final analysis, an interaction term for group x protocol based on the change in bladder-diary record keeping between the groups was included in multivariable models for the primary outcome analysis and was not significant. Control group participants completed bladder diaries and a daily exercise of mirrored-shape drawing21. Mirrored-shape drawing is a motor task of the upper extremities involving implicit memory that would not impact urinary symptoms and was meant to provide a motor skill exercise similar to the motor skill learning involved in performing pelvic floor muscle exercises. Participants were instructed to practice the mirrored-shape drawing for up to 15 minutes daily. Every 2 weeks, the participants practiced a different shape progressing from a square to a triangle to a pentagon and finally a star. The third and fourth in-person visits occurred at 2- and 4-weeks post-randomization. The interventionist provided one telephone check-in during the sixth week and then a final fifth visit occurred in-person at 8-weeks post-randomization.
Intervention Group
Participants randomized to the intervention group were taught to perform isolated pelvic floor muscle exercise contraction without abdominal muscle recruitment during a rectal or vaginal exam by the interventionist (nurse practitioner or PI) in the same visit after randomization. Participants were provided with information about fluid management (decrease caffeine, drink 6 to 8 eight-ounce glasses of fluid daily) and education regarding constipation management as indicated (increase physical activity, fiber, fruit, and fluid; over-the-counter agent use if needed). Participants were instructed to perform 45 pelvic floor muscle contractions and relaxations daily, divided into 3 sets of 15 with one set in each of 3 positions (lying, sitting, and standing).
At the third visit (2 weeks after randomization), participants were taught an urge suppression strategy, which involved teaching them to respond adaptively to the sensation of urgency. Those participants with stress-related urine loss were also instructed to squeeze pelvic floor muscles immediately prior to precipitants of leakage (sneezing, coughing, or bending). For those with nocturia (awakening from sleep at night to void), participants were instructed to remain lying in bed and perform the urge suppression strategy before arising from bed to void either in a bedside urinal (for men) or toilet. Similar to the control group, the third and fourth in-person visits occurred at 2- and 4-weeks post-randomization. The interventionist provided one telephone check-in during the sixth week and then a final fifth visit occurred in-person at 8-weeks post-randomization.
Baseline Characteristics
Participants self-reported race/ethnicity, level of completed education, and year of diagnosis of Parkinson’s disease. The presence of hypertension and diabetes were queried during the baseline examination and considered present if the patient reported they had been told by a doctor of a diagnosis or if they were currently prescribed medical therapy for the condition. Prostate enlargement was noted either on exam or with prescription of a medication used to treat prostate enlargement such as an alpha-blocker or 5-alpha reductase inhibitor. Anticholinergic medications included antimuscarinic bladder relaxants, tricyclic antidepressants, antihistamines, and antiparkinsonian medications with anticholinergic effects such as amantadine, trihexaphenidyl, or ethopropazine.
Outcome Evaluation
Outcomes were assessed at baseline and 8-weeks post-randomization. Primary outcome measures included weekly urinary incontinence frequency as reported on a 7-day bladder diary and OAB symptom burden as measured by the International Consultation on Incontinence OAB symptom questionnaire22. Bladder diary abstraction was performed by a trained research assistant who was blind to group allocation and did not interact with participants. To determine the clinical significance of any change observed in the ICIQ-OAB symptom questionnaire, the ICIQ-OAB bother and quality of life modules22 were completed at baseline and 8-weeks post-randomization.
Statistical Analysis
The primary outcome measures of weekly urinary incontinence frequency and ICIQ-OAB symptom score were assessed with intention to treat using generalized linear models adjusted for baseline severity according to the outcome measure of interest. Weekly urinary incontinence frequency was calculated using the baseline 7-day diary and 8-week post-randomization 7-day diary. If the participant completed at least 5 days of the 7-day diary, then the weekly frequency was adjusted to reflect a 7-day frequency. Because, eleven percent of participants dropped out with all six of these participants in the control group, sensitivity analyses were conducted using multiple imputation and last observation carried forward to account for differential dropout, which represented non-random missing data. Sensitivity analyses for the primary outcome of urinary incontinence frequency did not demonstrate differential results; however, differential results were obtained for the ICIQ-OAB symptom score. Using last observation carried forward, the analysis comparing the 8-week post-randomization ICIQ-OAB symptom score between the groups demonstrated statistical difference (behavioral group improved more than control), but no statistically significant difference was detected using the multiple imputation or completers analyses. The multiple imputation analyses are presented. Analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
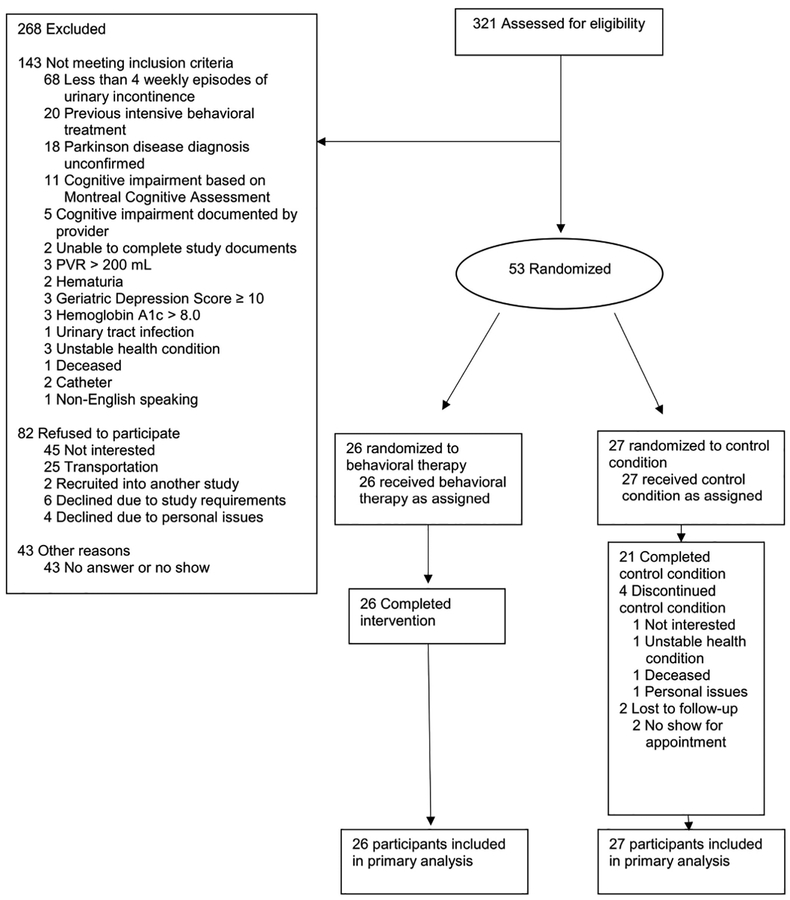
Screening and recruitment of participants is shown in Figure 1. The most common reason for not qualifying for the study was not experiencing at least 4 episodes of urinary incontinence during the screening period using the 7-day bladder diary. The randomization groups were well balanced across all baseline characteristics (Table 1). Participants were majority male, which reflects the Veteran population and the higher prevalence of Parkinson’s disease in males compared to females. The average MDS-UPDRS part 3 motor score suggests most participants had mild to moderate motor symptom severity in a defined ‘on’ period and the average disease duration was 6–8 years. The average MoCA score of approximately 24/30 suggests a substantial portion of participants had evidence of at least mild cognitive dysfunction. On average, participants reported 2 episodes of urinary incontinence per day.
Figure 1:
Study Participants
Table 1:
Baseline characteristics
| Control Group (n=27) |
Behavioral Group (n=26) |
p-value | |
|---|---|---|---|
| Age (years) | 69.7 ± 8.2 | 71.0 ± 6.1 | 0.5 |
| Sex | 21 (78%) male | 18 (70%) male | 0.5 |
| Race | 22 (88%) white 2 (8%) black 1 (4%) other 2 did not report |
22 (85%) white 3 (11%) black 1 (4%) other |
0.9 |
| Education | Completed High School or less 12% Completed College or more 72% |
Completed High School or less 30% Completed College or more 57% |
0.1 |
| Hypertension | 16 (59%) | 12 (46%) | 0.3 |
| Diabetes | 3 (11%) | 3 (11%) | 1.0 |
| Prostate Enlargement (men only) | 8/21 (38%) | 10/18 (56%) | 0.3 |
| Constipation (past/present) | 18 (67%) | 20 (77%) | 0.4 |
| Acetylcholinesterase Inhibitor | 1 (5%) | 4 (15%) | 0.2 |
| Anticholinergic medication | 7 (26%) | 6 (23%) | 0.8 |
| Parkinson disease duration (years) | 8.6 ± 5.0 | 6.1 ± 5.2 | 0.08 |
| MDS-UPDRS Part 3 | 23.6 ± 15.4 | 25.8 ± 13.2 | 0.6 |
| MoCA | 24.9 ± 2.4 | 23.5 ± 3.1 | 0.07 |
| Post-void residual (mL) | 30.3 ± 41.3 | 49.4 ± 53.6 | 0.2 |
| ICIQ-OAB symptom score | 8.3 ± 2.2 | 8.9 ± 2.4 | 0.3 |
| Weekly UI episodes (bladder diary) | 15.1 ± 11.1 | 13.9 ± 9.6 | 0.6 |
MDS-UPDRS = Movement Disorder Society Unified Parkinson Disease Rating Scale part 3 motor score
MoCA=Montreal Cognitive Assessment: lower score indicates greater impairment
International Consultation on Incontinence Overactive Bladder (ICIQ-OAB) Symptom Score: higher score indicates greater symptom frequency, range 0–16
UI=Urinary Incontinence
After randomization there were no dropouts in the behavioral therapy group, while 6 participants dropped out in the control group (11% of the overall study population). Characteristics of these six participants are listed in Supplementary Table 1. All participants who dropped out were male and tended to report more constipation, have a history of diabetes, and worse cognitive function.
Both groups reported reduction in weekly urinary incontinence episodes reported on a 7-day bladder diary at the 8-week post-randomization visit and there was no difference between the groups (Table 2). Participants in both groups also reported improvement in symptoms of overactive bladder as measured by the ICIQ-OAB symptom questionnaire and differences were not significantly different between groups (Table 2). This improvement was only clinically significant as evidenced by significant reduction in symptom bother and improvement in quality of life related to overactive bladder in the behavioral treatment group compared to the control group (Table 2).
Table 2:
Comparison of baseline and 8-week post-randomization frequency of urinary incontinence and ICIQ-OAB symptom, bother and quality of life scores by group
| Baseline | 8 weeks Post-Randomization | p-value *Between group |
|
|---|---|---|---|
|
Urinary Incontinence (bladder diary) |
0.89 | ||
| Control | 15.1 ± 11.1 | 8.5 ± 10.0 | |
| Behavioral | 13.9 ± 9.6 | 7.7 ± 10.5 | |
| ICIQ-OAB Symptom Score | 0.18 | ||
| Control | 8.3 ± 2.2 | 6.4 ± 2.4 | |
| Behavioral | 8.9 ± 2.4 | 5.8 ± 2.7 | |
| ICIQ-OAB Bother Score | 0.048 | ||
| Control | 27.8 ± 7.6 | 21.1 ± 6.8 | |
| Behavioral | 29.1 ± 8.3 | 16.6 ± 11.7 | |
| ICIQ-OAB QoL Score | 0.036 | ||
| Control | 74.7 ± 23.8 | 69.7 ± 23.8 | |
| Behavioral | 81.0 ± 24.2 | 58.4 ± 21.5 |
International Consultation on Incontinence Overactive Bladder (ICIQ-OAB) Symptom Score: higher score indicates greater symptom frequency, range 0–16
Bother score: higher score indicates greater bother related to overactive bladder symptoms, range 0–40
Quality of Life: higher score indicates worse quality of life, range 0–160
P-value: *Between group represents GLM adjusted for randomization group and baseline severity with multiple imputation for missing data
The sample size was too small for meaningful subgroup analyses; however, exploratory analyses revealed no statistical difference in 8-week overactive bladder symptom score or urinary incontinence frequency in the behavioral therapy group based upon level of cognitive function as measured by the Montreal Cognitive Assessment. Six-month outcomes were available for a subsample of participants (16/26) randomized to behavioral therapy who chose to provide data. Among these 16 individuals, the diary-reported baseline weekly urinary incontinence frequency of 13.2 ± 8.9 improved to 4.2 ± 9.7 at 8-weeks and was sustained at 6.2 ± 9.3 at 6 months (no statistically significant difference between 8 weeks and 6 months).
With regard to adherence to the pelvic floor muscle exercise regimen, at 8 weeks, 65% of participants in the behavioral therapy group reported performing the recommended number of exercises each day. At 6 months, 79% of those who provided data reported performing pelvic floor muscle exercises at least once daily and only 5% reported never doing exercises.
Discussion
Urinary symptoms, particularly urinary incontinence, add to the burden of living with Parkinson’s disease and non-motor symptoms have more impact on quality of life than motor symptoms as the disease progresses1. Clinical trial results to guide urinary symptom management in the setting of Parkinson’s disease are scant9. While behavioral therapy is well-established for adults with overactive bladder symptoms14,15,23, it has not been previously tested in a randomized controlled trial in persons with a neurodegenerative disease such as Parkinson’s disease. These results demonstrate that behavioral therapy is a reasonable initial treatment strategy in men and women with Parkinson’s disease, including those with mild impairment in cognition.
The results of this study suggest that self-monitoring with a daily bladder diary may lead to clinically significant improvement in the frequency of urinary incontinence and overactive bladder symptoms. The pathogenesis of urinary symptoms in Parkinson’s disease is hypothesized to be affected by two pathways involved in the underlying pathophysiology of the disease. Loss of dopaminergic balance in the basal ganglia leads to a loss of inhibition of bladder contractility through the pontine micturition center resulting in more frequent involuntary bladder contractions2. Additionally, cortical alpha-synuclein pathology may impact integration of sensory input from the bladder to the cortex leading to loss of recognition of bladder fullness and lack of advanced planning for the need to void (executive dysfunction)2. Daily bladder diaries have been associated with reduction in urinary incontinence frequency in previous studies; however the percent reduction was previously much less than participants assigned to multicomponent behavioral therapy14. Self-monitoring may have a particularly robust effect in the setting of a disease such as Parkinson’s disease associated with executive dysfunction24. While behavioral therapy was not superior when compared to a control group using bladder diary self-monitoring alone for urinary incontinence in the setting of Parkinson’s disease, the multicomponent nature of behavioral therapy likely provides strategies that have greater impact on bother and perhaps greater durability for long-term implementation than continued self-monitoring with a bladder-diary. Our preliminary data showing maintenance of the effect of behavioral therapy to reduce incontinence over six months are promising. No six-month effectiveness data were collected in the control group since they were promised behavioral treatment at 8 weeks as an inducement to participate in this randomized clinical trial.
Previous studies of behavioral therapy have excluded persons with evidence of cognitive impairment as measured using a screening test of cognitive function14,23. The present study is unique in its inclusion of persons who likely have at least mild cognitive dysfunction. It is not known what level of cognitive dysfunction precludes the ability to learn and implement exercise-based behavioral therapy. The most common alternative to behavioral therapy is drug therapy and most bladder relaxant therapy has the potential for anticholinergic side effects, which could impact cognitive function. Newer, more invasive therapies such as percutaneous tibial nerve stimulation or cystoscopic onobotulinumtoxin injection are associated with either multiple clinic visits for therapy or the need for sedation during a procedure25. Given these caveats to other therapeutic options, it is reasonable for clinicians to offer behavioral therapy as an initial treatment strategy in persons who are interested even in the presence of cognitive dysfunction. Indeed, other behavioral strategies such as prompted voiding may be viable even in persons with significant impairment if a care partner is available to assist.
Limitations to the current study include the short-term follow-up and majority male study population. However, the duration of follow-up is typical of many previous behavioral and drug trials for urinary symptoms and data were available from a subset in the behavioral therapy group who chose to provide 6-month follow-up data. While the study population was largely male, Parkinson’s disease is more prevalent in males. Additionally, with relatively few exclusion criteria, participants are reflective of many patients seen in the clinical setting, including those on stable medication regimens for Parkinson’s disease and urinary symptoms and those with evidence of at least mild cognitive dysfunction and use of acetylcholinesterase therapy. Outcomes in this study are patient-reported, which is the standard for clinical trials of treatments for urinary symptoms. Differential dropout in the control group led to non-random missing data, which could have impacted the ability to detect a difference in OAB symptom frequency between the groups. Next steps include a comparative effectiveness trial to compare behavioral therapy to commonly used drug therapy for urinary symptoms in Parkinson’s disease as well as strategies to make behavioral therapy accessible even if specialized continence care is not available.
Conclusions
Behavioral treatment is associated with improvement in urinary symptoms of overactive bladder in persons with Parkinson’s disease and multicomponent behavioral treatment is associated with reduced bother and improvement in quality of life. Given the favorable side effect profile of behavioral therapy compared to other treatment options such as drug therapy or other interventions, it is reasonable for clinicians to offer behavioral therapy as a first-line treatment option to persons with Parkinson’s disease. Evidence of mild cognitive dysfunction is not a barrier to implementation of exercise-based behavioral therapy for urinary symptom treatment. Self-monitoring using a bladder diary leads to improvement in urinary symptoms in the setting of Parkinson’s disease, which may reflect the impact of executive dysfunction in the pathogenesis of urinary incontinence in this population.
Supplementary Material
Acknowledgements
Sponsor’s role: Funding provide by a United States Department of Veterans Affairs Rehabilitation Research & Development career development award IK2 RX 000747–01 and the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs. The sponsors had no role in the design, methods, subject recruitment, data collection, analysis or preparation of the manuscript.
Footnotes
Conflicts of interest: The authors report no conflicts of interest
Trial Registration: clinicaltrials.gov Identifier:
The study was approved by the Institutional Review Boards at Emory University, the Birmingham VA Medical Center, and the University of Alabama at Birmingham and the Research and Development Committees at the Birmingham and Atlanta VA Medical Centers.
References
- 1.Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov Disord. 2010;25(15):2493–2500. [DOI] [PubMed] [Google Scholar]
- 2.McDonald C, Winge K, Burn DJ. Lower urinary tract symptoms in Parkinson’s disease: Prevalence, aetiology and management. Parkinson Relat Disord. 2017;35:8–16. [DOI] [PubMed] [Google Scholar]
- 3.Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N. Falls in outpatients with Parkinson’s disease: frequency, impact and identifying factors. J Neurol. 2005;252:1310–1315. [DOI] [PubMed] [Google Scholar]
- 4.Sakushima K, Yamazaki S, Fukuma S, et al. Influence of urinary urgency and other urinary disturbances on falls in Parkinson’s disease. J Neurol Sci. 2016;360:153–157. [DOI] [PubMed] [Google Scholar]
- 5.Fink H, Kuskowski M, Taylor B, et al. Association of Parkinson’s disease with accelerated bone loss, fractures and mortality in older men: the Osteoporotic Fractures in Men (MrOS) study. Osteoporos Int. 2008;19(9):1277–1282. [DOI] [PubMed] [Google Scholar]
- 6.Tanji H, Anderson KE, Gruber-Baldini AL, et al. Mutuality of the marital relationship in Parkinson’s disease. Mov Disord. 2008;23(13):1843–1849. [DOI] [PubMed] [Google Scholar]
- 7.Luppa M, Luck T, Weyerer S, König H-H, Brähler E, Riedel-Heller SG. Prediction of institutionalization in the elderly. A systematic review. Age Ageing. 2010;39(1):31–38. [DOI] [PubMed] [Google Scholar]
- 8.Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson’s disease—an evidence-based medicine review. Movement Disorders. 2019;34(2):180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zesiewicz TA, Evatt M, Vaughan CP, et al. Randomized, controlled pilot trial of solifenacin succinate for overactive bladder in Parkinson’s disease. Parkin Relat Disord. 2015;21(5):514–520. [DOI] [PubMed] [Google Scholar]
- 10.Cooper JA, Sagar HJ, Doherty SM, Jordan N, Tidswell P, Sullivan EV. Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson’s disease. A follow-up study of untreated patients. Brain. 1992;115(Pt 6):1701–1725. [DOI] [PubMed] [Google Scholar]
- 11.Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in Parkinson’s disease: a cohort study. J Neurol Neurosurg Psych. 2010;81(2):160–165. [DOI] [PubMed] [Google Scholar]
- 12.Crispo JAG, Willis AW, Thibault DP, et al. Associations between Anticholinergic Burden and Adverse Health Outcomes in Parkinson Disease. PLoS ONE. 2016;11(3):e0150621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goode PS, Burgio KL, Richter HE, Markland AD. Incontinence in older women. JAMA. 2010;303(21):2172–2181. [DOI] [PubMed] [Google Scholar]
- 14.Burgio KL, Locher JL, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA. 1998;280(23):1995–2000. [DOI] [PubMed] [Google Scholar]
- 15.Burgio KL, Goode PS, Johnson TM, et al. Behavioral Versus Drug Treatment for Overactive Bladder in Men: The Male Overactive Bladder Treatment in Veterans (MOTIVE) Trial. J Am Geriatr Soc. 2011;59(12):2209–2216. [DOI] [PubMed] [Google Scholar]
- 16.Vaughan CP, Juncos JL, Burgio KL, Goode PS, Wolf RA, Johnson TM 2nd. Behavioral therapy to treat urinary incontinence in Parkinson disease. Neurology. 2011;76(19):1631–1634. [DOI] [PubMed] [Google Scholar]
- 17.Nasreddine Z, Phillips N, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 18.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheikh J, Yesavage J. Geriatric Depression Scale (GDS): Recent evidence of development of a shorter version Clinical Gerontology: A Guide to Assessment and Intervention. New York: Haworth Press; 1986. [Google Scholar]
- 20.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 21.Howard Hughes Medical Institute. 2018; https://www.hhmi.org/biointeractive/classroom-activities-mirror-tracing-activity. Accessed March 11, 2019.
- 22.International Consultation on Incontinence. 2019; http://www.iciq.net/validationprotocol.htm. Accessed March 11, 2019.
- 23.Johnson TM II, Vaughan CP, Goode PS, et al. Pilot Results from a Randomized Trial in Men Comparing Alpha-Adrenergic Antagonist versus Behavior and Exercise for Nocturia and Sleep. Clin Ther. 2016;38(11):2394–2406.e2393. [DOI] [PubMed] [Google Scholar]
- 24.Kitta T, Kakizaki H, Furuno T, et al. Brain activation during detrusor overactivity in patients with Parkinson’s disease: a positron emission tomography study. J Urol. 2006;175(3 Pt 1):994–998. [DOI] [PubMed] [Google Scholar]
- 25.Kabay S, Canbaz Kabay S, Cetiner M, et al. The Clinical and Urodynamic Results of Percutaneous Posterior Tibial Nerve Stimulation on Neurogenic Detrusor Overactivity in Patients With Parkinson’s Disease. Urology. 2016;87:76–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.