Fig. 3.
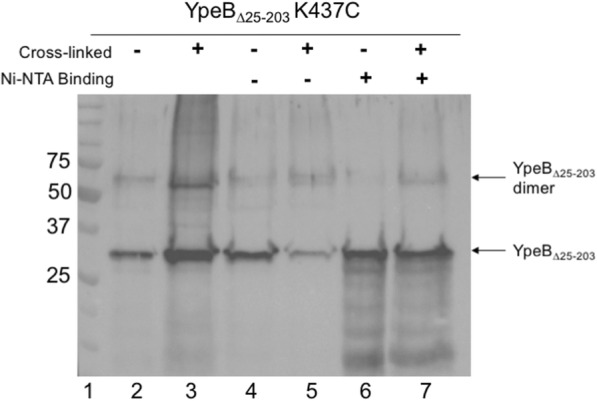
Column-bound YpeB∆25–203 K437C cross-linked complex. Two hundred optical density units of decoated dormant spores were cross-linked with APB. Cross-linked spores were lyophilized and mechanically broken. Proteins were extracted with 8 M urea binding buffer for 2 h. Spore lysates (lanes 2–3) were then passed over a Ni2+ NTA column to isolate YpeB∆25–203-His6 in addition to those proteins covalently bound via cross-links. Flow-thru (lanes 4–5) and bound (lanes 6–7) fractions were visualized via western blot using anti-YpeB antibodies [20]. The positions of protein size markers (lane 1) are indicated on the left. YpeB∆25–203 K437C monomers and dimers were detected in both the load (lane 3) and bound (lane 7) fractions of cross-linked spore samples
