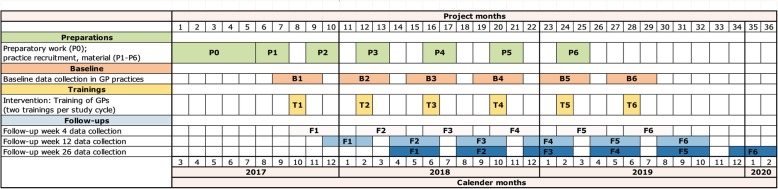
Fig. 1.
Gantt chart of the study illustrating the schedule of the total study, including the preparation period, pilot testing of the intervention, the distribution of the six study cycles (intervention) over the total duration of the study, and all periods of data collection. P0 Process evaluation pilot study, finalisation of training and manual, ethical approval, study registration. P1-P6 Preparations study cycles 1 to 6: practice recruitment, material, application for CME certification, scheduling of trainings. B1-B6 Pre- (7 days) and post-baseline (7 days) data collection of primary and secondary outcomes (S1-S3) in 8 GP practices per study cycle. T1-T6 Intervention: 3.5-h training of GPs in delivering brief stop-smoking advice according to either 5A or ABC. F1-F6 Postal follow-up data collection of secondary outcomes (S4-S9) corresponding to study cycle 1–6