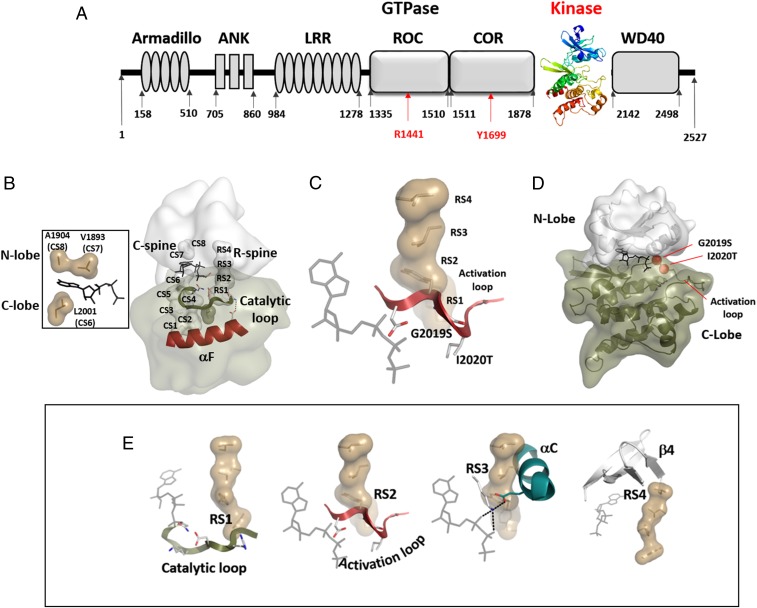
Fig. 1.
Identification of kinase spines in LRRK2. (A) Domain composition of LRRK2 highlighting the kinase domain as a central hub for LRRK2 regulation. (B) Spine residues in LRRK2 were identified based on a structural model of SRC1. The C-spine (CS1 to CS8) and the R-spine (RS1 to RS4) are connecting the N lobe (light gray) and the C lobe (dark green) and are bridged via the αF helix (red). The C-spine can only be completed by the recruitment of ATP (Left). (C and D) The magnesium-positioning loop contains the DYGψ motif, which encompasses 2 of the most common PD-related mutations (G2019S and I2020T). This loop is a hotspot for disease mutations, with D2017 being essential for orientating the γ-phosphoryl of ATP in most protein kinases. (E) The R-spine connects several highly conserved motifs in the kinase core. RS1 is part of the catalytic loop, including the HRD motif (YRD in LRRK2), while RS2 is an anchor of the AL. RS3 is orientating the αC helix. The highly flexible DYG motif as well as the αC helix are stabilized by R-spine formation via hydrophobic interactions between RS2, RS3, and RS1, with RS4 belonging to the β-strand 4 of the N lobe.