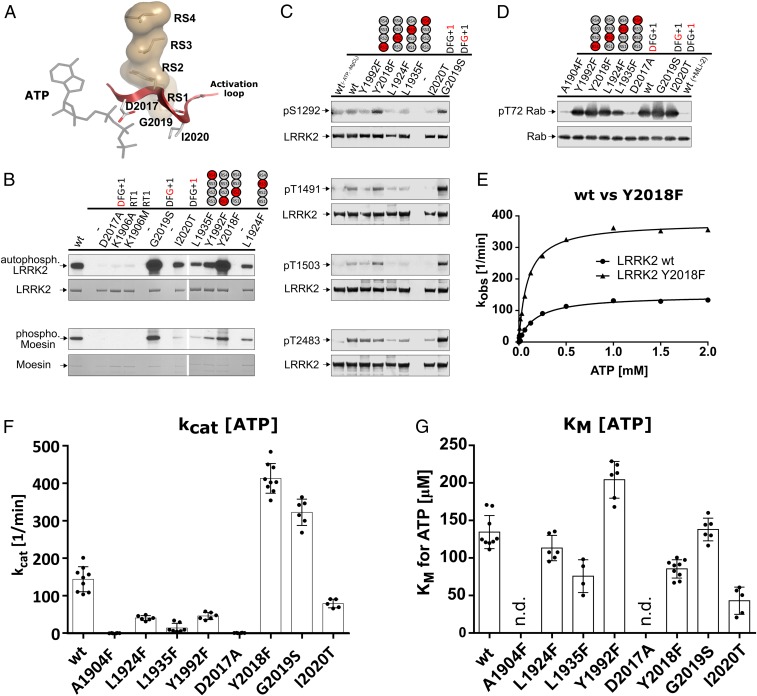
Fig. 3.
In vitro kinase assays render the LRRK2 R-spine mutation Y2018F (RS2) hyperactive. (A) The R-spine of LRRK2 connects 2 of the most flexible motifs in kinases, the αC helix via RS3 (L1924) and the AL (red) via RS2 (Y2018). (B) Based on kinase assays (Moesin) and autophosphorylation using [γ32P]-ATP, Y2018F (RS2F) was identified as a hyperactive kinase variant beside G2019S. All other R-spine mutations showed less phosphorylation. (C) Increased autophosphorylation was also revealed by Western blot analyses for Y2018F (RS2F) at positions S1292 and T1503 but not for T1491. G2019S, however, showed increased signals for all tested autophosphorylation sites. Y1992F (RS1F), L1924F (RS3F), and L1935F (RS4F) showed reduced autophosphorylation. (D) Reduced kinase activity for these mutants (Y1992F [RS1F], L1924F [RS3F], L1935F [RS4F]) was also found in an in vitro kinase assay with His-Rab8a (6-175). In contrast, Y2018F (RS2F), G2019S, and I2020T showed slightly enhanced phosphorylation of T72 in Rab8a. Rab8a phosphorylation was negligible for A1904F (CS8F), D2017A (DYG), and for the wt in the presence of 500 nM MLi-2. (E) Michaelis–Menten kinetics (raw data are in SI Appendix, Fig. S3 C and D) again demonstrate hyperactivity of Y2018F based on LRRKtide phosphorylation in a mobility shift assay. (F and G) KM(ATP) and kcat values from Michaelis–Menten kinetics using LRRKtide as substrate. All other tested spine mutations displayed reduced kinase activity.