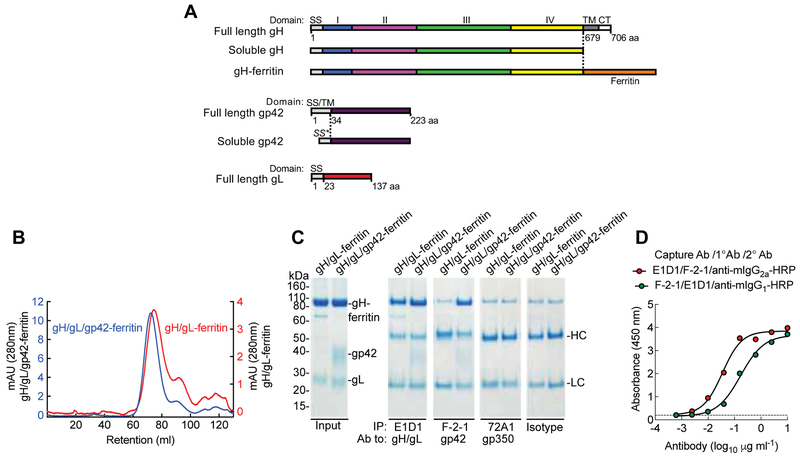
Figure 2.
Construction and characterization of gH/gL-ferritin and gH/gL/gp42-ferritin nanoparticles. (A) Schematic representation of full-length gH, soluble gH, gH-ferritin, full-length gp42, soluble gp42, and full-length gL. gH-ferritin fusion protein was generated by fusion of gH ectodomain (domains I, II, III, and IV) to the N-terminus of the ferritin. SS is the native signal sequence of the glycoprotein, SS* is the human CD5 signal sequence, TM is the transmembrane domain and CT is the cytoplasmic tail. Amino acid (aa) position of gH, gL, and gp42 is indicated. (B) Chromatograph from size-exclusion chromatography of gH/gL-ferritin and gH/gL/gp42-ferritin nanoparticles purified from the supernatants of mammalian cells transfected with gH-ferritin and gL plasmids or gH-ferritin, gL, and gp42 plasmids. Particles were affinity purified using snowdrop lectin prior to size exclusion chromatography. Transient transfection of gH-ferritin/gL and gH-ferritin/gL/gp42 plasmids, resulted in comparable amount of nanoparticle proteins (~ 2mg/L). (C) Characterization of nanoparticles by immunoprecipitation and SDS-PAGE. Bands corresponding to gH-ferritin, gp42, and gL are indicated. Purified nanoparticles were immunoprecipitated with anti-gH/gL mAb (E1D1), anti-gp42 mAb (F-2–1), anti-gp350 mAb (72A1), or isotype control antibody. HC and LC denote antibody heavy and light chains, respectively. Faint bands running slightly slower than gH-ferritin are nonspecific background bands. (D) Sandwich ELISA using mAb E1D1 or F-2–1 to capture purified gH/gL/gp42-ferritin nanoparticles and detection with mAb F-2–1 or E1D1, respectively. The dotted line represents the background. See also Figure S2 and S3.