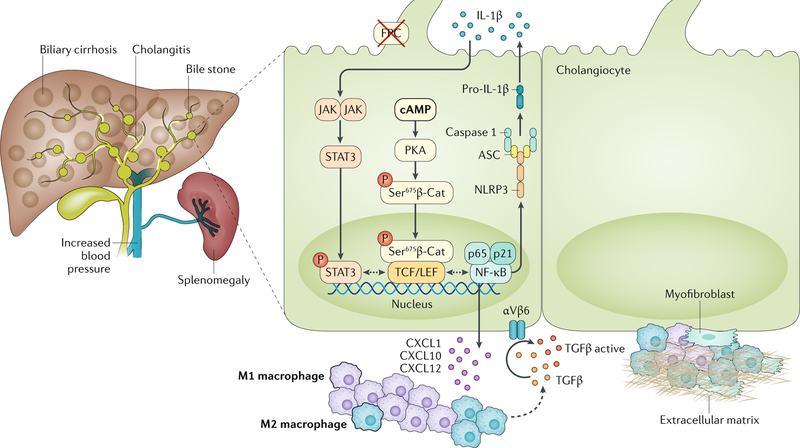
Fig. 3 |. Novel mechanisms of biliary fibrosis in ARPKD.
Liver disease in autosomal recessive polycystic kidney disease (ARPKD), which is caused by mutations in the PKHD1 gene (encoding fibrocystin (FPC)), is characterized by cystic dysgenesis of the intrahepatic bile ducts that retain an immature phenotype reminiscent of the embryonic biliary structures (ductal plate malformations). The disease is associated with progressive portal fibrosis, leading to portal hypertension and related complications. In FPC-defective cholangiocytes, increased levels of cAMP activate protein kinase A (PKA)-dependent phosphorylation of β-catenin (β-Cat) at Ser675 that leads to the nuclear translocation of pSer675β-catenin and transcriptional activation. This mechanism mediates the secretion of CXC-chemokine ligand 1 (CXCL1), CXCL10 and CXCL12 that recruit inflammatory cells, mostly M1 and then M2 macrophages, around the cystic epithelium. Macrophages secrete transforming growth factor-β (TGFβ) and TNF and stimulate the expression of αVβ6 integrins on cystic cholangiocytes that in turn activate latent TGFβ. CXCL10 secretion is further increased by the production of IL-1β through the activation of the Janus kinase (JAK)–signal transducer and activator of transcription 3 (STAT3) pathway. IL-1β secretion is mediated by an activated inflammasome. Signalling molecules that are druggable are highlighted in bold and detailed in TABLE 2. ASC, apoptosis-associated speck-like protein containing a CARD; NF-κB, nuclear factor-κB; NLRP3, NOD-, LRR- and pyrin domain-containing 3.