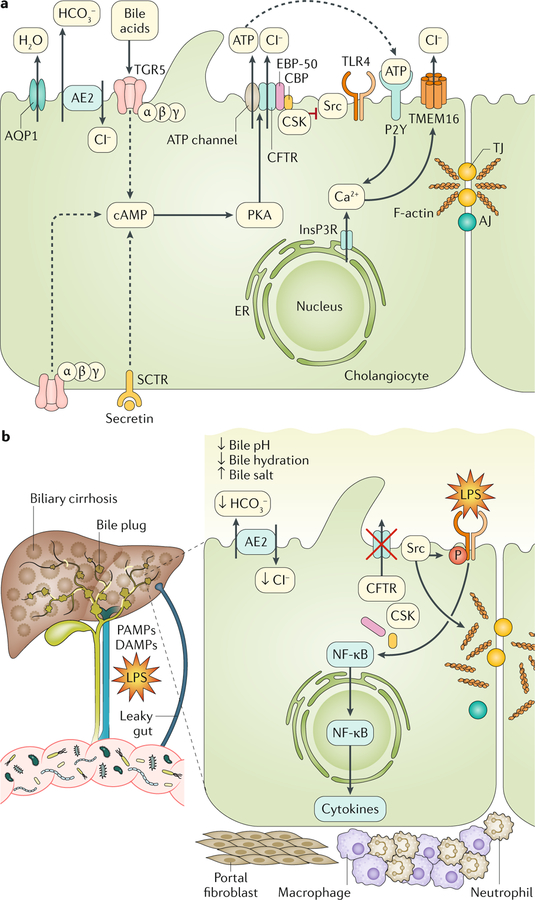
Fig. 4 |. CFTR function in cholangiocytes.
a | Cystic fibrosis transmembrane conductance regulator (CFTR) is located on the apical membrane of cholangiocytes where it has a major role in modifying bile properties (fluidity and pH). Bicarbonate (HCO3−) secretion into bile is necessary to sustain bile flow, to enable clearance of xenobiotics and to accomplish digestive needs (digestion and absorption of fats) within the intestine. Secretin is the main hormone that controls the secretory functions of the biliary epithelium. Secretin interacts with the G protein-coupled secretin receptor (SCTR) expressed on the basolateral membrane of cholangiocytes and triggers the production of cAMP, which activates protein kinase A (PKA). In turn, PKA phosphorylates the R domain of CFTR and opens the chloride conductivity channel. Apical chloride secretion is mediated by bicarbonate exchange through the anion exchanger 2 (AE2), generating an electrolyte–osmotic gradient that favours the passive movement of water through aquaporins (AQPs). These mechanisms are responsible for the normal hydration and alkalization of bile. CFTR-dependent biliary secretion is also triggered in response to TGR5 receptor–PKA signalling when TGR5 is stimulated by bile acids. TGR5, a membrane-bound bile acid receptor coupled to a stimulatory G protein, is expressed in the liver by several non-parenchymal cells (including the sinusoidal epithelium, Kuppfer cells and hepatic stellate cells) and by cholangiocytes. On cholangiocytes, TGR5 is localized both on the apical membrane and on the primary cilium and is strongly activated by taurolithocholic acid and taurocholate. In vitro data in mouse cholangiocytes have shown that CFTR can mediate the apical release of ATP that binds to P2Y purinergic receptors on the apical membrane of biliary cells and activates the Ca2+–calmodulin chloride channel (TMEM16). CFTR also regulates the function of proteins involved in biliary innate immunity and endotoxin tolerance (such as Src family tyrosine kinase and its regulatory proteins CBP and CSK). b | Mutations in the gene encoding CFTR cause a chronic cholangiopathy that can eventually progress to biliary cirrhosis. When CFTR is absent, biliary secretion is impaired and the protein complex formed with Src and its regulatory proteins is disrupted, resulting in the self-activation of Src. Src activation is responsible for the phosphorylation of Toll-like receptor 4 (TLR4), which enhances its response to lipopolysaccharide (LPS) and increases nuclear factor-κB (NF-κB) activation and cytokine secretion. This inflammatory milieu also affects the F-actin cytoskeleton and tight junction (TJ) integrity, altering the epithelial barrier function. CFTR loss also promotes changes in the gut that favour the colonization of a more pro-inflammatory microbiota and the translocation of their products to the liver. The altered biliary epithelial innate immunity and changes in the gut microbiota synergistically lead to the progression of cystic fibrosis-related liver disease. Signalling molecules that are druggable are detailed in TABLE 2. AJ, adherens junction; DAMP, damage-associated molecular pattern; ER, endoplasmic reticulum; InsP3R, inositol-1,4,5-trisphosphate receptor; PAMP, pathogen-associated molecular pattern.