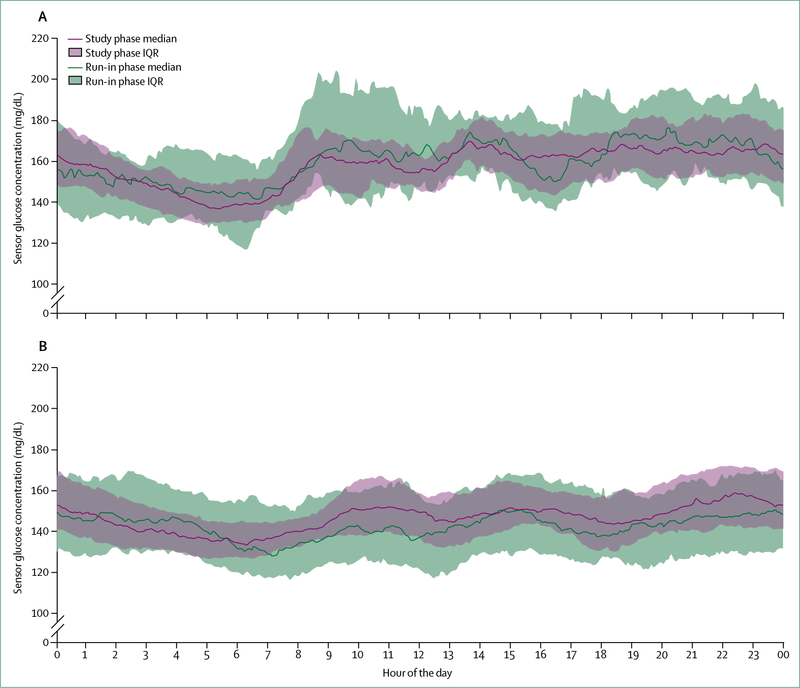
Figure 5: Glucose concentrations in patients with type 1 diabetes with a hybrid closed-loop system, before and after use, over 24 h.
Sensor glucose profiles from 124 people with type 1 diabetes, of which 30 were adolescents (14–21 years; A) and 94 were adults (22–75 years; B), before (during run-in phase) and during the study phase using the Medtronic MiniMed 670 g hybrid closed-loop system (Medtronic, Northridge CA, USA) under clinical trial conditions. Median and IQR of sensor glucose values are given as a green line and band for the run-in phase, and a pink line and band for the study phase, respectively. In the run-in phase, the hybrid closed-loop system was in manual mode, with participants making all treatment decisions except for the pump automatically suspending before senor glucose concentrations became too low. In the study phase, the hybrid closed-loop system was in auto mode. Participants had less variability in their blood glucose concentration during auto mode. Reproduced from Garg et al,92 with permission from Mary Ann Liebert.