Abstract
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) is an inherited small vessel disease that leads to early cerebrovascular events and functional disability. It is the most common single-gene disorder leading to stroke. Magnetic resonance imaging (MRI) is a central component of the diagnosis and monitoring of CADASIL. Here we provide a descriptive review of the literature on three important aspects pertaining to the use of MRI in CADASIL. First, we review past research exploring MRI markers for this disease. Secondly, we describe results from studies investigating associations between neuroimaging abnormalities and neuropathology in CADASIL. Finally, we discuss previous findings relating MRI markers to clinical symptoms. This review thus provides a summary of the current state of knowledge regarding the use of MRI in CADASIL as well as suggestions for future research.
Keywords: CADASIL, Magnetic Resonance Imaging, Neuroimaging, Diagnosis, Biomarkers
Introduction
Cerebral small vessel disease (SVD) is a broad term used to describe consequences of pathological processes affecting small vessels of the brain [57]. SVD is a significant underlying cause of ischemic strokes and intracerebral hemorrhage. It is also major contributor to dementia, mood disorders, gait disturbances and disability [49]. SVD is found in high frequency in the elderly population and is usually sporadic in nature. However, inherited forms of SVD exist, including cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL).
CADASIL is a unique type of SVD caused by mutations in the NOTCH3 gene [29]. This disorder is characterized by the occurrence of subcortical ischemic events, such as transient ischemic attacks (TIA) and strokes, at early age and often in the absence of typical risk factors for cerebrovascular disease [6, 28]. It is recognized as the most prevalent monogenic cause of stroke and vascular dementia. The pathological hallmarks of CADASIL include the degeneration of vascular smooth muscle cells and pericytes as well as the presence of granular osmiophilic material (GOM) within vessels [6, 28]. Macroscopic examinations of affected brain tissues have revealed the presence of diffuse myelin rarefaction, lacunes mostly affecting subcortical areas, and cortical apoptosis [6, 28]. CADASIL presents with various neurological and psychiatric symptoms including migraines with aura, cognitive impairment, gait abnormality and mood disturbance [6]. Although the clinical presentation of CADASIL varies considerably between affected individuals, the mean age of onset of clinical symptoms is around 35 to 40 years [14, 17].
Genetic testing is the gold standard for the clinical diagnosis of CADASIL. Additionally, the clinical investigation generally involves the combined examination of clinical, metabolic and radiological characteristics, together with a review of family history [14]. Magnetic Resonance Imaging (MRI) is a central component of the diagnosis of CADASIL and is routinely used to document SVD in patients [66]. To provide an overview of the utility of MRI in CADASIL, this review article summarizes the past scientific literature on three main aspects relevant to this topic: 1) the use of MRI in the diagnosis of CADASIL; 2) the associations between MRI markers and pathological processes in CADASIL and 3) the associations between MRI markers and clinical manifestations of CADASIL.
1). The use of MRI in the diagnosis of CADASIL
The core MRI abnormalities observed in CADASIL have been described in several reports and include the presence of white matter hyperintensities (WMH), subcortical infarcts and cerebral microbleeds (CM) [66]. The radiological presentation of CADASIL varies considerably between affected individuals [14, 17]. Likely contributing to this variability, growing evidence suggests that the clinical and radiological presentations in CADASIL are dependent on the genotype [26, 35, 44–46, 61, 62]. While MRI abnormalities appear at a variable age in CADASIL, they can be observed in the vast majority of patients aged above 35 years [6]. Age is an important factor in predicting the extent of brain alterations in patients and is positively correlated with the prevalence and severity of changes observed on MRI [7, 19]. It has further been estimated that MRI signal irregularities in CADASIL can be detected 10–15 years prior to the onset of clinical manifestations [6]. MRI signal abnormalities in asymptomatic individuals are less severe and less diffuse than in symptomatic patients, suggesting an association between the severity of these abnormalities and increasing symptomatology [6]. T2-weighted images, a type of sequence often used to detect the presence of pathology and ischemic events, appears to be more sensitive than T1-weighted images to early manifestations of CADASIL. Accordingly, the earliest reported MRI changes in CADASIL consist in areas of increased signal on T2-weighted or fluid-attenuated inversion recovery (FLAIR) images, frequently in the periventricular white matter [6]. In an attempt to characterize the neuroimaging characteristics of CADASIL, studies have contrasted MRI findings from NOTCH3 mutation carriers and age-matched non-carriers [15, 25, 27, 38, 43, 51, 58, 65, 70, 76]. In CADASIL patients, these studies have reported the presence of WMH predominantly in the periventricular region, anterior temporal pole, external capsule as well as frontal and parietal areas. The pattern of lesions is often described as symmetrical. White matter in the posterior temporal and occipital lobes, the basal ganglia, the thalamus, the pons and the internal capsule is considerably less affected. Arcuate fibers, also known as cortical association or U-shape fibers, are generally spared. Involvement of the corpus callosum, which is rare in sporadic forms of SVD, has been described in CADASIL individuals, but often in a small proportion of cases. A higher frequency of dilated perivascular space (PVS), measured on T2 images using pre-established criteria, has also been highlighted in this population [13, 70]. The vast majority of structural MRI studies performed CADASIL patients have exposed the presence of diffuse and regional brain atrophy [31, 34, 60, 67]. In contrast, a previous study on members of a single family carrying a CADASIL mutation concluded that atrophy was rather rare [12]. This discrepant finding can be explained by methodological limitations, including the use of a small number of patients with a genetically confirmed diagnosis of CADASIL and a subjective assessment of brain atrophy. Diffusion tensor imaging (DTI), a MRI technique providing an estimate of the microstructural integrity of cerebral white matter, is of great potential relevance to the field of CADSIL. Using DTI, studies have described important diffusion changes in the white matter of CADASIL patients [8, 47, 55]. Significant differences in histograms representing the whole-brain trace of the diffusion have been observed between CADASIL patients and age-matched controls, pointing to the presence of widespread microstructural tissue damage in this disease [48]. The microstructural integrity of the basal nuclei and the thalamus appears to be particularly affected [47, 55]. To aid the differential diagnosis, an important focus has been given to the description of specific MRI markers allowing to distinguish CADASIL from other conditions presenting similar clinical and/or radiological features (e.g. sporadic SVD or multiple sclerosis). Studies comparing genetically confirmed CADASIL patients to non-carriers presenting with CADASIL-like presentations reveal largely similar imaging characteristics between groups [1–3, 27, 51, 58, 68]. Previous evidence suggests that anterior temporal lobe involvement on MR images is highly specific and allows for distinguishing CADASIL from other conditions [1, 2, 10, 27, 43, 52, 54, 64, 68–70, 76]. For example, O’Sullivan et al. (2001) reported a sensitivity of 90% and specificity of 100% of anterior temporal WMH to differentiate CADASIL from sporadic forms of leukoaraiosis [54]. A different study highlighted a sensitivity of 89% and specificity of 86% for “moderate” or “severe” anterior temporal pole WMH in the diagnosis of CADASIL [43]. These results are in agreement with a study from Van den Boom et al. (2003) demonstrating that the presence of WMH in the anterior temporal lobe was the only MRI feature consistently seen in the youngest CADASIL patients (aged 20–30 years) [69]. The presence of lesions in the external capsule has also been proposed as a potential diagnostic feature of CADASIL [1, 3, 9, 10, 43, 52, 54, 58, 64, 65, 76], although it has been reported in a lower proportion of patients and has been associated with a lower diagnostic specificity [69]. Despite the identification of MRI differences between CADASIL patients and non-carriers, these differences are overall limited and not consistently found across studies. MRI features described as specific to CADASIL, including temporal pole WMH, have been observed in a considerable proportion of subjects without NOTCH3 mutations, limiting their specificity [51, 58, 63]. Previous findings also demonstrate that these features may be absent at early stages of the disease [64, 67]. Adding to the complexity of establishing specific MRI markers for CADASIL, several studies indicate that the MRI presentation of patients may vary depending on the genotype. For example, anterior temporal involvement appears to be less common in subjects with cysteine-sparing NOTCH3 mutations, although the role of cysteine-sparing mutations in the pathogenesis of CADASIL remains a matter of debate [26, 35, 44–46, 62]. As such, at the present time, CADASIL cannot be reliably differentiated from other forms of SVD on the sole basis of MRI. While the involvement of the anterior temporal pole and external capsule may hint the presence of CADASIL, these features are not sufficient to confirm its diagnosis. This emphasizes the importance of considering additional information in the diagnostic process.
2). Associations between MRI markers and pathological processes in CADASIL
Studies investigating relationships between neuropathology and MRI markers provide valuable information on pathological processes underlying MRI signal abnormalities. As such, they allow a more valid and comprehensive interpretation of MRI findings. Only a few studies have systematically investigated these relationships in CADASIL patients. Dichgans and colleagues (2002) attempted to link in-vivo MRI to autopsy findings [19]. MRI scans were acquired in 16 CADASIL patients, while postmortem pathological examinations were performed on seven different patients. Because none of the autopsied cases had available MRI data, this study was mostly observational and relationships between imaging and pathological variables were not directly assessed. Autopsy findings revealed lacunes and diffuse white matter changes resulting from demyelination, axonal loss, gliosis, and extracellular space enlargement. The authors further described focal accumulations of hemosiderin-containing macrophages in six of the seven autopsied brains. They hypothesized that homogenous rounded foci of signal loss apparent on T2*-weighted images likely corresponded to hemosiderin deposits after the occurrence of CM, although other causes could not be excluded due to methodological limitations. Viswanathan et al. (2006) studied neuronal apoptosis in four CADASIL patients who died from complication of the disease and had an MRI performed the year prior to death [72]. Apoptotic neurons were observed in all cases at autopsy. Neuronal apoptosis was not found within or close to cortical microinfarcts. Subsequent analyses showed that the number of apoptotic neurons in layers 3 and 5 was associated with the extent of subcortical WMH and axonal damage. In two patients with available quantitative MRI data, the authors observed that the patient with more severe apoptosis also presented greater volumes of WMH and lacunes. The severity of the apoptosis was related to normalized brain volumes, suggesting that it likely contributes to cerebral atrophy in CADASIL. In a single case study, Jouvent et al. (2011) studied a 53 year-old CADASIL patient using both postmortem neuropathological examination and high-resolution 7-T MRI (HR-MRI) [32]. The results of this combined investigation showed that hypointensities of a linear shape with regular edges, of a few hundred micrometers in diameter, crossing the cortical mantle on consecutive slices on T2* images, corresponded to microvessels. The authors further identified two subtypes of intracortical infarcts, as confirmed by histological examination. On T2* images, these infarcts were observed as small hypointense foci of irregular shape and of signal intensity similar to that of white matter. Some of these lesions were circular and did not reach the edges of the cortical mantle, whereas others were pyramidal with their bases resting on the gray/white matter border. Both types of lesions were present across all cerebral lobes. The authors argued that the use of high-resolution MRI could contribute to the detection of intracortical infarcts, which can be difficult based solely on pathological examination or low-resolution MRI. Iron deposition has been proposed as a biomarker for various neuropathological processes, including cerebral small vessel disease. For this reason, Liem et al. (2012) investigated the presence of iron deposition in relation to small vessel disease in three CADASIL patients using combined high-resolution 7-T MRI and histopathological examination of postmortem brains [39]. Histochemistry revealed the presence of iron deposition in the caudate nucleus, putamen and, to a lesser degree, in the globus pallidus. The observed pattern of iron accumulation matched the pattern of signal loss on postmortem MRI. The authors conclude that MRI signal hypointensity is linked to progressive iron accumulation. Yamamoto et al. (2009) simulated MR images by juxtaposing digital pictures of serial in-vitro slices of the temporal pole from a single CADASIL patient [74]. They proposed that MRI temporal pole hyperintensities in CADASIL patients reflect enlarged PVS, together with myelin depletion and axonal damage. Using indirect measures, other studies have reported links between pathology and MRI signal abnormalities in CADASIL. For examples, in both CADASIL and sporadic small-vessel disease patients, Duering (2018) demonstrated significant associations between serum neurofilament light chain (a blood marker for axonal damage) and measures of brain volume, WMH, lacunes and CM [22]. Studies have also relied on high-resolution 7-T MR technologies to estimate underlying pathological processes in CADASIL. Liem et al. (2010) used 7-T MR angiography to characterize the luminal diameters of lenticulostriate arteries in-vivo [41]. Luminal diameters were unaffected in CADASIL patients. The authors found no associations between luminal diameters and lacunes in the basal ganglia, suggesting that these lesions were not caused by a narrowing of lenticulostriate arteries. De Guio et al. (2014) measured the white matter venous density in CADASIL patients using HR 7-T MRI [16]. They showed a reduction in the density of visible venous vasculature in both normal appearing white matter and WMH. Fang et al. (2017) examined changes in retinal vessel using Enhanced Depth Imaging Optical Coherence Tomography in relation to 7-T MRI markers [24]. They found moderate, but significant, correlations between the presence of CM or small infarcts and measures of retinal vessels integrity. Taken together, these results support the validity of MRI investigation in the detection of pathological alterations in CADASIL patients. Future systematic studies combining MRI and examinations of post-mortem tissues to characterize pathological mechanisms underlying MRI signal abnormalities in CADASIL patients could promote the optimization of MRI sequences to detect and classify brain lesions in-vivo.
3). The associations between MRI markers and clinical manifestations of CADASIL
To identify and validate MRI markers for CADASIL, the characterization of associations between these markers and clinical symptoms or disease progression is crucial. For this purpose, a large number of studies have explored clinical correlates of MRI abnormalities in CADASIL. The overall lesion load observed on T1-weighted MR images has been associated with the degree of global disability, often quantified using the modified Rankin Scale [4], and with performance on different cognitive domains [18, 76]. Conversely, O’Sullivan (2004) failed to find significant correlations between the total lesion load on T2-weighted images and global cognition, suggesting that the examination of specific types of lesions might be of greater interest [55]. Accordingly, numerous studies have instead focused on the associations with specific MRI markers of CADASIL. As described in the previous section, the presence of WMH is a common and early MRI feature of CADASIL. Studies investigating relationships between WMH and clinical symptoms have produced mixed results. While the severity of WMH has been linked to the presence and severity of depressive symptomatology in CADASIL patients [59, 64], most studies highlight a lack of independent influence of WMH on cognitive performance and disability [36, 71, 73]. Congruently, in a 7-year follow-up study, changes in WMH volume over time was not associated with decline in global cognition [37]. A potential factor contributing to the lack of association between WMH and cognitive or functional outcomes consists in the great variability in the spatial distribution of WMH across patients. A recent study from Duchesnay (2018) highlighted distinct regional patterns of WMH, each associated with different clinical outcomes [20]. According to the authors, considering regional WMH burden, rather than adopting a whole-brain approach, allowed a superior prediction of clinical outcomes. As such, previous studies measuring the global WMH burden might have underestimated its clinical relevance. Cerebral microbleeds is another central neuroimaging features of CADASIL. Studies examining relationships between CM and clinical symptoms have also reached mixed findings. Several studies have outlined significant associations between the number of CM and indices of disease progression, including advancing age [56], greater global disability [11, 72, 73], lower score on the mini-mental state examination (MMSE) [72] and poorer performance in executive function [5]. The increase in the number of CM over time has further been linked with a decrease in global cognitive functioning, memory and executive function [37]. In opposition, other studies have failed to find an independent influence of CM on cognition [56, 73] or depressive symptoms [59]. Potentially contributing to discrepancies in findings across studies, it has been proposed that CM may not have a direct effect on disability in CADASIL but rather represent a consequence of the severity of other lesions [72]. In contrast with WMH or CM, a larger volume of lacunes has been consistently linked with poorer clinical outcomes or cognitive performances in CADASIL patients [11, 36, 40, 50, 53, 59, 71, 73]. In a longitudinal study, the baseline volume of lacunes was found to predict subsequent global cognitive functioning and disability [30]. Furthermore, increases in lacunes over time have been associated with a worsening of performance in executive function [37, 42]. As such, the burden of lacunes has been proposed as the most relevant MRI maker with regards to cognitive impairments in CADASIL [36, 40, 73]. Brain atrophy is a well-documented consequence of CADASIL. The vast majority of studies investigating associations between clinical symptoms and global volumetric brain measures, such as the brain parenchymal fraction (BPF), have revealed significant relationships with cognitive scores and functional outcomes in CADASIL patients [34, 50, 53, 60, 71]. Using multimodal imaging in a large cohort, Viswanathan et al. (2010) established that brain atrophy corresponded to the main determinant of disability and global cognitive function in CADASIL [71]. In comparison, Benisty et al. (2012) failed to find significant correlations between scores on multiple cognitive measures and the BPF [5]. However, this group studied a specific CADASIL subpopulation presenting without lacunes, potentially contributing to this divergence in findings. Baseline brain volumes, or changes in brain volumes over time, also appear to contribute to the prediction of subsequent cognitive functioning and disability [30, 42, 60]. While they did not find significant associations with brain atrophy, Liem et al. (2009) revealed strong correlations between ventricular enlargement, likely secondary to central cortical atrophy, and decline in global cognitive functioning over time [37]. Looking at regional patterns of brain atrophy, a study demonstrated significant hippocampal volume reduction in patients meeting clinical criteria for dementia, as opposed to non-demented patients [53]. Hippocampal volumes were also found to independently predict scores on a global cognitive performance scale. In two articles looking at regional morphometric characteristics of CADASIL patients, Jouvent et al. showed strong correlations between sulci morphology and clinical measures of apathy, global cognitive functioning and disability [31, 33]. Multiple studies have explored associations between white matter tract integrity, as estimated via DTI, and clinical symptoms in CADASIL [8, 47, 48, 55, 71]. Overall, these studies support significant correlations between measures of disability or global cognitive functioning and diffusion parameters. O’Sullivan (2004) et al. noted particularly strong associations between executive function performance and DTI measurements, one of the most salient cognitive impairment in CADASIL [55]. Additionally, Molko et al. (2002) reported larger changes in whole-brain diffusion parameters in patients experiencing clinical deterioration over time than in patients maintaining a stable status [48]. Finally, more uncommon MRI markers have been studied in relation to clinical symptoms in CADASIL. For example, Yao et al. (2014) demonstrated that the severity of PVS dilatation was worsened in demented CADASIL patients and was associated with global cognitive functioning, regardless of the age [75]. In a different study, the presence of intracranial atherosclerosis, as estimated with MR angiography, was independently linked to increased disability [11]. Moreton et al. (2018) found that CADASIL patients with lower cerebral vasoreactivity, as estimated using Arterial Spin Labeling (ASL), tended to present greater levels of disability, depressive symptoms and impaired processing speed [50]. To summarize, many studies have related MRI markers to clinical symptoms in CADASIL. However, these results are at times inconsistent across studies and need replication. In this literature, the presence and extent of lacunes and brain atrophy has been the most consistently associated with cognitive and/or functional outcomes in CADASIL. Yet, certain factors merit further consideration. For example, in two articles, Duering et al. proposed that the regional pattern of distribution of MRI lesions is an important factor in the explanation of observed deficits [21, 23]. Furthermore, in agreement with the notion of cognitive reserve, the education level appears to mediate the relationship between MRI lesions and clinical symptoms in CADASIL [77]. To provide a better understanding of the clinical relevance of MRI signal abnormalities in CADASIL, factors influencing the relationship between MRI markers and the presentation or progression of symptoms need to be investigated more thoroughly.
Conclusion
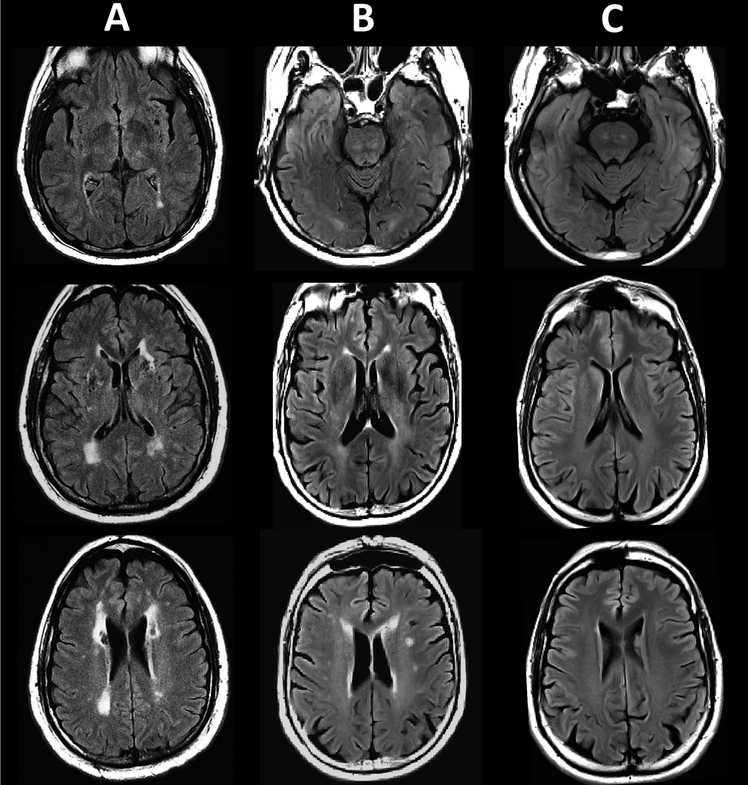
Magnetic resonance imaging is a core component of the clinical diagnosis of CADASIL, a genetic condition leading to early cerebrovascular changes and strokes. Here, we reviewed and summarized the literature on three aspects relevant to the use of MRI in CADASIL. Although the imaging characteristics of CADASIL have been previously described in a review article [66], the present review adds to the existing literature by further exploring relationships between MRI markers and clinical or pathological features of CADASIL. The review of studies investigating potential MRI markers for CADASIL indicates that it shares largely similar radiological features with other diseases affecting white matter integrity, such as multiple sclerosis or sporadic forms of SVD (as an illustration, see Figure 1). Accordingly, while the involvement of the anterior temporal pole and external capsule can suggest the presence of CADASIL, none of the proposed MRI markers is sufficiently sensitive or specific to support the differential or accurate diagnosis of this disease. This review also points to the lack of research systematically examining associations between MRI markers and pathology in CADASIL. This type of work is needed to obtain a better understanding of pathogenic mechanisms underlying MRI signal abnormalities and may promote the development of more precise imaging biomarkers for this disease. Finally, the review of studies investigating relationships between MRI markers and clinical symptoms in CADASIL indicates that lacunes and brain atrophy are the most robustly related to disability and cognitive status. However, associations between clinical and imaging variables appear to fluctuate depending on the type of symptoms examined as well as other modulating factors, such as the cognitive reserve or the regional distribution of brain lesions. Future research is needed to better characterize factors influencing the interplay between radiological and clinical presentations in CADASIL, including the effects of the genotype or various lifestyle/environmental factors. A thorough understanding of factors associated with neuroimaging findings in CADASIL is necessary to promote accurate diagnosis, improve disease management and facilitate the validation of new therapeutic strategies.
Figure 1.
Fluid-attenuated inversion recovery (FLAIR) images of white matter signal abnormalities in: A) a 61 year old male subject with CADASIL (image courtesy of Dr. Anand Viswanathan, J. Philip Kistler Stroke Research Center, Massachusetts General Hospital, Boston, USA); B) a 58 male subject with sporadic cerebral small vessel disease (SVD) and C) a 58 male subject with normal appearing white matter.
Acknowledgements
D.S. postdoctoral fellowship is partly funded by the Fonds de Recherche Santé Québec (Canada). J.F.A.V. is funded by UH3 NS100121 and RF1 NS110048 grants from National Institute of Neurological Disorders and Stroke. Y.T.Q. and H.T.T. are funded by the Massachusetts General Hospital Executive Committee on Research (ECOR).
Footnotes
Declarations of interest
None.
REFERENCES
- [1].Abramycheva N, Stepanova M, Kalashnikova L, Zakharova M, Maximova M, Tanashyan M, Lagoda O, Fedotova E, Klyushnikov S, Konovalov R, New mutations in the Notch3 gene in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL), Journal of the neurological sciences 349 (2015) 196–201. [DOI] [PubMed] [Google Scholar]
- [2].Auer DP, Putz B, Gossl C, Elbel G-K, Gasser T, Dichgans M, Differential lesion patterns in CADASIL and sporadic subcortical arteriosclerotic encephalopathy: MR imaging study with statistical parametric group comparison, Radiology 218 (2001) 443–451. [DOI] [PubMed] [Google Scholar]
- [3].Ayrignac X, Carra-Dalliere C, Menjot de Champfleur N, Denier C, Aubourg P, Bellesme C, Castelnovo G, Pelletier J, Audoin B, Kaphan E, Adult-onset genetic leukoencephalopathies: a MRI pattern-based approach in a comprehensive study of 154 patients, Brain 138 (2014) 284–292. [DOI] [PubMed] [Google Scholar]
- [4].Banks JL, Marotta CA, Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis, Stroke 38 (2007) 1091–1096. [DOI] [PubMed] [Google Scholar]
- [5].Benisty S, Reyes S, Godin O, Hervé D, Zieren N, Jouvent E, Zhu Y, During M, Dichgans M, Chabriat H, White-matter lesions without lacunar infarcts in CADASIL, Journal of Alzheimer’s Disease 29 (2012) 903–911. [DOI] [PubMed] [Google Scholar]
- [6].Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser M-G, Cadasil, The Lancet Neurology 8 (2009) 643–653. [DOI] [PubMed] [Google Scholar]
- [7].Chabriat H, Levy C, Taillia H, Iba-Zizen M-T, Vahedi K, Joutel A, Tournier-Lasserve E, Bousser M-G, Patterns of MRI lesions in CADASIL, Neurology 51 (1998) 452–457. [DOI] [PubMed] [Google Scholar]
- [8].Chabriat H, Pappata S, Poupon C, Clark C, Vahedi K, Poupon F, Mangin J, Pachot-Clouard M, Jobert A, Le Bihan D, Clinical severity in CADASIL related to ultrastructural damage in white matter: in vivo study with diffusion tensor MRI, Stroke 30 (1999) 2637–2643. [DOI] [PubMed] [Google Scholar]
- [9].Chabriat H, Vahedi K, Bousser M, Iba-Zizen M, Joutel A, Nibbio A, Nagy T, Lasserve ET, Krebs M, Julien J, Clinical spectrum of CADASIL: a study of 7 families, The Lancet 346 (1995) 934–939. [DOI] [PubMed] [Google Scholar]
- [10].Chawda S, De Lange R, Hourihan M, Halpin S, Clair DS, Diagnosing CADASIL using MRI: evidence from families with known mutations of Notch 3 gene, Neuroradiology 42 (2000) 249–255. [DOI] [PubMed] [Google Scholar]
- [11].Choi JC, Song S-K, Lee JS, Kang S-Y, Kang J-H , Diversity of stroke presentation in CADASIL: study from patients harboring the predominant NOTCH3 mutation R544C, Journal of Stroke and Cerebrovascular Diseases 22 (2013) 126–131. [DOI] [PubMed] [Google Scholar]
- [12].Coulthard A, Blank S, Bushby K, Kalaria R, Burn D, Distribution of cranial MRI abnormalities in patients with symptomatic and subclinical CADASIL, The British journal of radiology 73 (2000) 256–265. [DOI] [PubMed] [Google Scholar]
- [13].Cumurciuc R, Guichard JP, Reizine D, Gray F, Bousser M, Chabriat H, Dilation of Virchow-Robin spaces in CADASIL, European journal of neurology 13 (2006) 187–190. [DOI] [PubMed] [Google Scholar]
- [14].Davous P, CADASIL: a review with proposed diagnostic criteria, European Journal of Neurology 5 (1998) 219–233. [DOI] [PubMed] [Google Scholar]
- [15].De Guio F, Reyes S, Vignaud A, Duering M, Ropele S, Duchesnay E, Chabriat H, Jouvent E, In vivo high-resolution 7 Tesla MRI shows early and diffuse cortical alterations in CADASIL, PloS one 9 (2014) e106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].De Guio F, Vignaud A, Ropele S, Duering M, Duchesnay E, Chabriat H, Jouvent E, Loss of Venous Integrity in Cerebral Small Vessel Disease, Stroke (2014). [DOI] [PubMed] [Google Scholar]
- [17].Desmond DW, Moroney JT, Lynch T, Chan S, Chin SS, Mohr J, The natural history of CADASIL: a pooled analysis of previously published cases, Stroke 30 (1999) 1230–1233. [DOI] [PubMed] [Google Scholar]
- [18].Dichgans M, Filippi M, Brüning R, Iannucci G, Berchtenbreiter C, Minicucci L, Uttner I, Crispin A, Ludwig H, Gasser T, Quantitative MRI in CADASIL correlation with disability and cognitive performance, Neurology 52 (1999) 1361–1361. [DOI] [PubMed] [Google Scholar]
- [19].Dichgans M, Holtmannspotter M, Herzog J, Peters N, Bergmann M, Yousry TA, Cerebral microbleeds in CADASIL: a gradient-echo magnetic resonance imaging and autopsy study, Stroke 33 (2002) 67–71. [DOI] [PubMed] [Google Scholar]
- [20].Duchesnay E, Selem FH, De Guio F, Dubois M, Mangin J-F, Duering M, Ropele S, Schmidt R, Dichgans M, Chabriat H, Different Types of White Matter Hyperintensities in CADASIL, Frontiers in neurology 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Duering M, Gonik M, Malik R, Zieren N, Reyes S, Jouvent E, Hervé D, Gschwendtner A, Opherk C, Chabriat H, Identification of a strategic brain network underlying processing speed deficits in vascular cognitive impairment, Neuroimage 66 (2013) 177–183. [DOI] [PubMed] [Google Scholar]
- [22].Duering M, Konieczny MJ, Tiedt S, Baykara E, Tuladhar AM, van Leijsen E, Lyrer P, Engelter ST, Gesierich B, Achmüller M, Serum neurofilament light chain levels are related to small vessel disease burden, Journal of stroke 20 (2018) 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Duering M, Zieren N, Hervé D, Jouvent E, Reyes S, Peters N, Pachai C, Opherk C, Chabriat H, Dichgans M, Strategic role of frontal white matter tracts in vascular cognitive impairment: a voxel-based lesion-symptom mapping study in CADASIL, Brain 134 (2011) 2366–2375. [DOI] [PubMed] [Google Scholar]
- [24].Fang X-J, Yu M, Wu Y, Zhang Z-H, Wang W-W, Wang Z-X, Yuan Y, Study of Enhanced Depth Imaging Optical Coherence Tomography in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy, Chinese medical journal 130 (2017) 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fattapposta F, Restuccia R, Pirro C, Malandrini A, Locuratolo N, Amabile G, Bianco F, Early diagnosis in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL): the role of MRI, Functional neurology 19 (2004) 239–242. [PubMed] [Google Scholar]
- [26].Ge W, Kuang H, Wei B, Bo L, Xu Z, Xu X, Geng D, Sun M, A novel cysteine-sparing NOTCH3 mutation in a Chinese family with CADASIL, PloS one 9 (2014) e104533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].He D, Chen D, Li X, Hu Z, Yu Z, Wang W, The comparisons of phenotype and genotype between CADASIL and CADASIL-like patients and population-specific evaluation of CADASIL scale in China, The journal of headache and pain 17 (2016) 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Herve D, Chabriat H, Cadasil, Journal of geriatric psychiatry and neurology 23 (2010) 269–276. [DOI] [PubMed] [Google Scholar]
- [29].Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cécillion M, Maréchal E, Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia, Nature 383 (1996) 707. [DOI] [PubMed] [Google Scholar]
- [30].Jouvent E, Duchesnay E, Hadj-Selem F, De Guio F, Mangin J-F, Hervé D, Duering M, Ropele S, Schmidt R, Dichgans M, Prediction of 3-year clinical course in CADASIL, Neurology 87 (2016) 1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jouvent E, Mangin J-F, Porcher R, Viswanathan A, O’sullivan M, Guichard J-P, Dichgans M, Bousser M-G, Chabriat H, Cortical changes in cerebral small vessel diseases: a 3D MRI study of cortical morphology in CADASIL, Brain 131 (2008) 2201–2208. [DOI] [PubMed] [Google Scholar]
- [32].Jouvent E, Poupon C, Gray F, Paquet C, Mangin J-F, Le Bihan D, Chabriat H, Intracortical infarcts in small vessel disease: a combined 7-T postmortem MRI and neuropathological case study in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy, Stroke 42 (2011) e27–e30. [DOI] [PubMed] [Google Scholar]
- [33].Jouvent E, Reyes S, Mangin J-F, Roca P, Perrot M, Thyreau B, Hervé D, Dichgans M, Chabriat H, Apathy is related to cortex morphology in CADASIL A sulcal-based morphometry study, Neurology 76 (2011) 1472–1477. [DOI] [PubMed] [Google Scholar]
- [34].Jouvent E, Viswanathan A, Mangin J-F, O’Sullivan M, Guichard J-P, Gschwendtner A, Cumurciuc R, Buffon F, Peters N, Pachaï C, Brain atrophy is related to lacunar lesions and tissue microstructural changes in CADASIL, Stroke 38 (2007) 1786–1790. [DOI] [PubMed] [Google Scholar]
- [35].Kim Y, Choi E, Choi C, Kim G, Choi J, Yoo H, Kim J, Characteristics of CADASIL in Korea A novel cysteine-sparing Notch3 mutation, Neurology 66 (2006) 1511–1516. [DOI] [PubMed] [Google Scholar]
- [36].Lee JS, Choi JC, Kang S-Y, Kang J-H, Na HR, Park J-K, Effects of lacunar infarctions on cognitive impairment in patients with cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy, Journal of Clinical Neurology 7 (2011) 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liem M, Oberstein SL, Haan J, Van der Neut I, Ferrari M, Van Buchem M, Middelkoop H, Van der Grond J, MRI correlates of cognitive decline in CADASIL A 7-year follow-up study, Neurology 72 (2009) 143–148. [DOI] [PubMed] [Google Scholar]
- [38].Liem MK, Lesnik Oberstein SA, Haan J, van der Neut IL, van den Boom R, Ferrari MD, van Buchem MA, van der Grond J, Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: progression of MR abnormalities in prospective 7-year follow-up study, Radiology 249 (2008) 964–971. [DOI] [PubMed] [Google Scholar]
- [39].Liem MK, Oberstein SAL, Versluis MJ, Maat-Schieman ML, Haan J, Webb AG, Ferrari MD, van Buchem MA, van der Grond J, 7 T MRI reveals diffuse iron deposition in putamen and caudate nucleus in CADASIL, J Neurol Neurosurg Psychiatry 83 (2012) 1180–1185. [DOI] [PubMed] [Google Scholar]
- [40].Liem MK, van der Grond J, Haan J, van den Boom R, Ferrari MD, Knaap YM, Breuning MH, van Buchem MA, Middelkoop HA, Lesnik Oberstein SA, Lacunar infarcts are the main correlate with cognitive dysfunction in CADASIL, Stroke 38 (2007) 923–928. [DOI] [PubMed] [Google Scholar]
- [41].Liem MK, van der Grond J, Versluis MJ, Haan J, Webb AG, Ferrari MD, van Buchem MA, Lesnik Oberstein SA, Lenticulostriate arterial lumina are normal in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy: a high-field in vivo MRI study, Stroke 41 (2010) 2812–2816. [DOI] [PubMed] [Google Scholar]
- [42].Ling Y, De Guio F, Jouvent E, Duering M, Hervé D, Guichard JP, Godin O, Dichgans M, Chabriat H, Clinical correlates of longitudinal MRI changes in CADASIL, Journal of Cerebral Blood Flow & Metabolism (2018) 0271678X18757875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Markus H, Martin R, Simpson M, Dong Y, Ali N, Crosby A, Powell J, Diagnostic strategies in CADASIL, Neurology 59 (2002) 1134–1138. [DOI] [PubMed] [Google Scholar]
- [44].Matsushima T, Conedera S, Tanaka R, Li Y, Yoshino H, Funayama M, Ikeda A, Hosaka Y, Okuzumi A, Shimada Y, Genotype–phenotype correlations of cysteine replacement in CADASIL, Neurobiology of aging 50 (2017) 169. e167–169. e114. [DOI] [PubMed] [Google Scholar]
- [45].Mazzei R, Conforti F, Lanza P, Sprovieri T, Lupo M, Gallo O, Patitucci A, Magariello A, Caracciolo M, Gabriele A, A novel Notch3 gene mutation not involving a cysteine residue in an Italian family with CADASIL, Neurology 63 (2004) 561–564. [DOI] [PubMed] [Google Scholar]
- [46].Mizuno T, Muranishi M, Torugun T, Tango H, Nagakane Y, Kudeken T, Kawase Y, Kawabe K, Oshima F, Yaoi T, Two Japanese CADASIL families exhibiting Notch3 mutation R75P not involving cysteine residue, Internal Medicine 47 (2008) 2067–2072. [DOI] [PubMed] [Google Scholar]
- [47].Molko N, Pappata S, Mangin J, Poupon C, Vahedi K, Jobert A, LeBihan D, Bousser M, Chabriat H, Diffusion tensor imaging study of subcortical gray matter in CADASIL, Stroke 32 (2001) 2049–2054. [DOI] [PubMed] [Google Scholar]
- [48].Molko N, Pappata S, Mangin J-F, Poupon F, LeBihan D, Bousser M-G, Chabriat H, Monitoring disease progression in CADASIL with diffusion magnetic resonance imaging: a study with whole brain histogram analysis, Stroke 33 (2002) 2902–2908. [DOI] [PubMed] [Google Scholar]
- [49].Moran C, Phan TG, Srikanth VK, Cerebral small vessel disease: a review of clinical, radiological, and histopathological phenotypes, International Journal of Stroke 7 (2012) 36–46. [DOI] [PubMed] [Google Scholar]
- [50].Moreton FC, Cullen B, Delles C, Santosh C, Gonzalez RL, Dani K, Muir KW, Vasoreactivity in CADASIL: Comparison to structural MRI and neuropsychology, Journal of Cerebral Blood Flow & Metabolism 38 (2018) 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nannucci S, Pescini F, Bertaccini B, Bianchi S, Ciolli L, Valenti R, Dotti M, Federico A, Inzitari D, Pantoni L, Clinical, familial, and neuroimaging features of CADASIL-like patients, Acta Neurologica Scandinavica 131 (2015) 30–36. [DOI] [PubMed] [Google Scholar]
- [52].O’Riordan S, Nor A, Hutchinson M, CADASIL imitating multiple sclerosis: the importance of MRI markers, Multiple Sclerosis Journal 8 (2002) 430–432. [DOI] [PubMed] [Google Scholar]
- [53].O’Sullivan M, Ngo E, Viswanathan A, Jouvent E, Gschwendtner A, Saemann PG, Duering M, Pachai C, Bousser M-G, Chabriat H, Hippocampal volume is an independent predictor of cognitive performance in CADASIL, Neurobiology of aging 30 (2009) 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].O’Sullivan M, Jarosz J, Martin R, Deasy N, Powell J, Markus H, MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL, Neurology 56 (2001) 628–634. [DOI] [PubMed] [Google Scholar]
- [55].O’Sullivan M, Singhal S, Charlton R, Markus HS, Diffusion tensor imaging of thalamus correlates with cognition in CADASIL without dementia, Neurology 62 (2004) 702–707. [DOI] [PubMed] [Google Scholar]
- [56].Oberstein SL, Van den Boom R, Van Buchem M, Van Houwelingen H, Bakker E, Vollebregt E, Ferrari M, Breuning M, Haan J, Cerebral microbleeds in CADASIL, Neurology 57 (2001) 1066–1070. [DOI] [PubMed] [Google Scholar]
- [57].Pantoni L, Gorelick PB, Cerebral small vessel disease, Cambridge University Press, 2014. [Google Scholar]
- [58].Pantoni L, Pescini F, Nannucci S, Sarti C, Bianchi S, Dotti M, Federico A, Inzitari D, Comparison of clinical, familial, and MRI features of CADASIL and NOTCH3-negative patients, Neurology 74 (2010) 57–63. [DOI] [PubMed] [Google Scholar]
- [59].Park JH, Jeon B-H, Lee JS, Newhouse PA, Taylor WD, Boyd BD, Kim KW, Kim M-D, CADASIL as a useful medical model and genetic form of vascular depression, The American Journal of Geriatric Psychiatry 25 (2017) 719–727. [DOI] [PubMed] [Google Scholar]
- [60].Peters N, Holtmannspötter M, Opherk C, Gschwendtner A, Herzog J, Sämann P, Dichgans M, Brain volume changes in CADASIL A serial MRI study in pure subcortical ischemic vascular disease, Neurology 66 (2006) 1517–1522. [DOI] [PubMed] [Google Scholar]
- [61].Rutten JW, Van Eijsden BJ, Duering M, Jouvent E, Opherk C, Pantoni L, Federico A, Dichgans M, Markus HS, Chabriat H, The effect of NOTCH3 pathogenic variant position on CADASIL disease severity: NOTCH3 EGFr 1–6 pathogenic variant are associated with a more severe phenotype and lower survival compared with EGFr 7–34 pathogenic variant, Genetics in Medicine (2018) 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Scheid R, Heinritz W, Leyhe T, Thal D, Schober R, Strenge S, von Cramon DY, Froster U, Cysteine-sparing notch3 mutations: cadasil or cadasil variants?, Neurology 71 (2008) 774–776. [DOI] [PubMed] [Google Scholar]
- [63].Schmidt H, Zeginigg M, Wiltgen M, Freudenberger P, Petrovic K, Cavalieri M, Gider P, Enzinger C, Fornage M, Debette S, Genetic variants of the NOTCH3 gene in the elderly and magnetic resonance imaging correlates of age-related cerebral small vessel disease, Brain 134 (2011) 3384–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Singhal S, Rich P, Markus HS, The spatial distribution of MR imaging abnormalities in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy and their relationship to age and clinical features, American Journal of Neuroradiology 26 (2005) 2481–2487. [PMC free article] [PubMed] [Google Scholar]
- [65].Skehan SJ, Hutchinson M, MacErlaine DP, Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: MR findings, American journal of neuroradiology 16 (1995) 2115–2119. [PMC free article] [PubMed] [Google Scholar]
- [66].Stojanov D, Aracki-Trenkic A, Vojinovic S, Ljubisavljevic S, Benedeto-Stojanov D, Tasic A, Vujnovic S, Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL), Bosnian journal of basic medical sciences 15 (2015) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Stromillo ML, Dotti MT, Battaglini M, Mortilla M, Bianchi S, Plewnia K, Pantoni L, Inzitari D, Federico A, De Stefano N, Structural and metabolic brain abnormalities in preclinical CADASIL, Journal of Neurology, Neurosurgery & Psychiatry (2008). [DOI] [PubMed] [Google Scholar]
- [68].Tomimoto H, Ohtani R, Wakita H, Lin J-X, Ihara M, Miki Y, Oshima F, Murata T, Ishibashi K, Suenaga T, Small artery dementia in Japan: radiological differences between CADASIL, leukoaraiosis and Binswanger’s disease, Dementia and geriatric cognitive disorders 21 (2006) 162–169. [DOI] [PubMed] [Google Scholar]
- [69].van den Boom R, Lesnik Oberstein SA, Ferrari MD, Haan J, van Buchem MA, Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: MR imaging findings at different ages—3rd–6th decades, Radiology 229 (2003) 683–690. [DOI] [PubMed] [Google Scholar]
- [70].van den Boom R, Lesnik Oberstein SA, van Duinen SG, Bornebroek M, Ferrari MD, Haan J, van Buchem MA, Subcortical lacunar lesions: an MR imaging finding in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, Radiology 224 (2002) 791–796. [DOI] [PubMed] [Google Scholar]
- [71].Viswanathan A, Godin O, Jouvent E, O’Sullivan M, Gschwendtner A, Peters N, Duering M, Guichard J-P, Holtmannspötter M, Dufouil C, Impact of MRI markers in subcortical vascular dementia: a multi-modal analysis in CADASIL, Neurobiology of aging 31 (2010) 1629–1636. [DOI] [PubMed] [Google Scholar]
- [72].Viswanathan A, Gray F, Bousser M-G, Baudrimont M, Chabriat H, Cortical neuronal apoptosis in CADASIL, Stroke 37 (2006) 2690–2695. [DOI] [PubMed] [Google Scholar]
- [73].Viswanathan A, Gschwendtner A, Guichard J-P, Buffon F, Cumurciuc R, O’Sullivan M, Holtmannspötter M, Pachai C, Bousser M-G, Dichgans M, Lacunar lesions are independently associated with disability and cognitive impairment in CADASIL, Neurology 69 (2007) 172–179. [DOI] [PubMed] [Google Scholar]
- [74].Yamamoto Y, Ihara M, Tham C, Low RW, Slade JY, Moss T, Oakley AE, Polvikoski T, Kalaria RN, Neuropathological correlates of temporal pole white matter hyperintensities in CADASIL, Stroke 40 (2009) 2004–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yao M, Hervé D, Jouvent E, Duering M, Reyes S, Godin O, Guichard JP, Dichgans M, Chabriat H, Dilated perivascular spaces in small-vessel disease: a study in CADASIL, Cerebrovascular Diseases 37 (2014) 155–163. [DOI] [PubMed] [Google Scholar]
- [76].Yousry TA, Seelos K, Mayer M, Brüning R, Uttner I, Dichgans M, Mammi S, Straube A, Mai N, Filippi M, Characteristic MR lesion pattern and correlation of T1 and T2 lesion volume with neurologic and neuropsychological findings in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), American journal of neuroradiology 20 (1999) 91–100. [PubMed] [Google Scholar]
- [77].Zieren N, Duering M, Peters N, Reyes S, Jouvent E, Hervé D, Gschwendtner A, Mewald Y, Opherk C, Chabriat H, Education modifies the relation of vascular pathology to cognitive function: cognitive reserve in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, Neurobiology of aging 34 (2013) 400–407. [DOI] [PubMed] [Google Scholar]