Abstract
Drug relapse after periods of abstinence is a common feature of substance abuse. Moreover, anxiety and other mood disorders are often co-morbid with substance abuse. Cholinergic receptors in the ventral tegmental area (VTA) are known to mediate drug-seeking and anxiety-related behavior in rodent models. However, it is unclear if overlapping VTA cholinergic mechanisms mediate drug relapse and anxiety-related behaviors associated with drug abstinence. We examined the effects of VTA cholinergic receptor blockade on cue-induced cocaine seeking and anxiety during cocaine abstinence. Male Sprague-Dawley rats were trained to self-administer intravenous cocaine (~ 0.5 mg/kg/infusion, FR1 schedule) for 10 days, followed by 14 days of forced abstinence. VTA infusion of the non-selective nicotinic acetylcholine receptor antagonist mecamylamine (0, 10, and 30 μg/sidc) or the non-selective muscarinic receptor antagonist scopolamine (0, 2.4 and 24 μg/side) significantly decreased cue-induced cocaine seeking. In cocaine naïve rats, VTA mecamylamine or scopolamine also led to dose-dependent increases in open arm time in the elevated plus maze (EPM). In contrast, rats that received I.V. cocaine, compared to received I.V. saline rats, displayed an anxiogenic response on day 14 of abstinence as reflected by decreased open arm time in the EPM. Furthermore, low doses of VTA mecamylamine (10 μg /side) or scopolamine (2.4 μg /side), that did not alter EPM behavior in cocaine naive rats, were sufficient to reverse the anxiogenic effects of cocaine abstinence. Together, these data point to an overlapping role of VTA cholinergic mechanisms to regulate relapse and mood disorder-related responses during cocaine abstinence.
INTRODUCTION
Drug addiction is a chronic and relapsing brain disease (Hyman et al., 2006; Fattore and Diana, 2016) characterized by an increased susceptibility to drug relapse during periods of drug abstinence (Shaham and Hope, 2005; Sinha, 2013). Drug addiction is also often co-morbid with mood-related disorders, including depression and anxiety (Conway et al., 2006). Moreover, symptoms of depression and anxiety during periods of drug abstinence can increase craving intensity and are reliable predictors of drug relapse (Back et al., 2010; Sinha et al., 2011; Schellekens et al., 2015; Fatseas et al., 2018). Indeed, higher depression and anxiety scores in drug abusers are associated with heightened reactivity to drug cues (Feldstein Ewing et al., 2010). Both human and preclinical rodent studies have highlighted a critical role of the mesolimbic dopamine (DA) system, including the ventral tegmental area (VTA) to nucleus accumbens (NAc) pathway, in cue-induced drug craving and drug seeking (Childress et al., 1999; Phillips et al., 2003; Shaham and Hope, 2005; Volkow et al., 2006; Solecki et al., 2013). More recently in rodent models, activity in the VTA to NAc pathway has also been shown to causally and bidirectionally regulate mood disorder related behavioral phenotypes in the tail suspension, forced swim test (FST), sucrose preference and chronic social defeat stress tests (Chaudhury et al., 2013; Tye et al., 2013; Friedman et al., 2014)
Prior work has shown that VTA acetylcholine receptors (AChRs) regulate drug cue-related behavior, including cue-induced drug seeking (Lof et al., 2007; Zhou et al., 2007; Solecki et al., 2013). Our recent data revealed that pharmacologically increasing cholinergic tone in the VTA is also sufficient to produce pro-depressive, anxiogenic-like and anhedonic-like behavioral responses. In contrast, blockade of VTA muscarinic acetylcholine receptors (mAChRs) or nicotinic acetylcholine receptors (nAChRs) produces an antidepressant-like effect in the FST (Addy et al., 2015a). However, it is unknown whether VTA cholinergic mechanisms mediate behaviors associated with comorbid substance use disorders and mood or anxiety disorders during cocaine abstinence. Here, we trained rats to self-administer intravenous (i.v.) cocaine or saline for 10 days followed by a 14-day period of forced abstinence, and then examined cue-induced drug-seeking behavior or anxiety-related behavior during abstinence. First, we determined whether VTA mAChR or nAChR blockade altered cue-induced cocaine-seeking behavior. Secondly, we examined whether cocaine abstinence led to anxiogenic response in the EPM. Finally, we investigated whether VTA AChR blockade altered EPM behavioral responses in cocaine naive and cocaine abstinent rats.
Our data, revealing the ability of AChR blockade to alter cocaine-seeking and EPM behavior, is an important addition demonstrating common VTA cholinergic mechanisms that mediate both substance abuse and anxiety-related behavior. In our rat model, we also found that cocaine self-administration and 14 days of forced abstinence produced an anxiogenic effect that was reversed by AChR blockade in the VTA. Thus, VTA mAChR or nAChR blockade, respectively, attenuated both cue-induced drug-relapse and abstinence-induced anxiogenic responses. Taken together, these findings have important implications regarding cholinergic receptors mechanisms that could serve as possible therapeutic targets for comorbid substance use and anxiety-related disorders.
EXPERIMENTAL PROCEDURES
Animals and surgery
A total of 167 adult male Sprague Dawley rats were used in this study. (250-279 g; Charles River Laboratories, Wilmington, MA, USA) Upon arrival, rats were doubly housed and allowed to acclimate to the housing facility for 7 days. Rats were maintained under a 12h light/dark cycle with climate control between 22-24°C. Food and water was available ad libitum unless noted otherwise. All experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Yale University Institutional Animal Care and Use Committee. All surgical procedures were performed using aseptic techniques. In preparation for surgery, rats were anesthetized with ketamine HC1 (100 mg/kg, i.p., Henry Schein, NY, USA) and xylazine (10 mg/kg, i.p., Henry Schein, NY, USA), and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). Prior to surgical incision, rats received administration of the long-acting nonsteroidal anti-inflammatory drug, carprofen (5 mg/kg s.c., Henry Schein, NY, USA) and were implanted with a bilateral cannula targeting the VTA and a chronic indwelling catheter in the right jugular vein, as previously described (McFarland and Kalivas, 2001; Solecki et al., 2013) All coordinates were obtained from the rat brain atlas (Paxinos and Watson, 2007) with anteroposterior (AP), mediolateral (ML) and dorsoventral (DV) positions referenced from Bregma. A bilateral cannula (Plastics One, Roanoke, VA, USA) spaced 1 mm apart was placed 1 mm above the VTA (AP −5.2 to −5.4 mm, ML ± 0.5 mm, DV −8.1 mm from skull) (Paxinos and Watson 2007) and secured to the skull using screws (Gexpro, High Point, NC, USA) and dental cement (Dentsply, Milford, DE, USA). After surgery, rats were singly housed and allowed to recover for 5–7 days before behavioral training and/or testing.
Intravenous cocaine and saline self-administration
Self-administration training started after 5-7 days of surgical recovery and was preceded by 2–3 days of food restriction to ~90% of free feeding body weight levels. Each rat received 2 h daily training sessions for 10 consecutive days and training was performed in standard operant chambers, illuminated by a house light (Med Associate, St. Albans, USA). Rats were trained under fixed ratio 1 (FR1) schedule of reinforcement during which each active lever press led to intravenous cocaine (0.18 mg over 6 s, ~0.5 mg/kg) or saline (0.9%) infusion and conditioned stimulus (CS) cue presentation (tone + stimulus light for 6 s), followed by a 6 sec timeout period, during which active lever pressing was recorded but resulted in no programmed consequence. Throughout training, inactive lever presses had no programmed consequence. Acquisition of stable cocaine self-administration behavior was defined as less than 15% variability in total active lever pressing over 3 consecutive days. At the completion of 10-day cocaine self-administration, catheter patency was assessed by infusing 200 μl of 2.5 mg/ml Brevital (Henry Schein, NY, USA) into the catheter and observing mild loss of muscle tone and the righting reflex. 18 rats were not responsive to the Brevital, reflecting a non-patent catheter and were eliminated from the study and from all analyses.
VTA drug administration
For intra-VTA drug infusion, bilateral internal cannula were inserted into the guide cannula and extended 1 mm beyond the guide to be in the VTA. Drugs were delivered in a 0.3μ1 volume infused over 2 min via a micro-infusion pump and syringe (25 gauge, Hamilton Syringe, Reno, NE, USA). After the 2 min infusion was complete, the internal cannulae were left in place for 1 min to allow for complete diffusion of the drug. The non-selective nicotinic and muscarinic receptor antagonists mecamylamine and scopolamine, respectively, were dissolved in 0.9% saline. The selection of doses for the mecamylamine (0, 10, and 30 μg) and scopolamine (0, 2.4, and 24 μg) were based on previously published data showing the behavioral effectiveness of these VTA infusion doses (Sharf et al., 2006; Schmidt et al., 2009; Solecki et al., 2013; Addy et al., 2015a). Importantly, we previously demonstrated that these behaviorally relevant doses had no effect on general locomotor activity (Solecki et al., 2013; Addy et al., 2015a).
Cue-induced cocaine-seeking test
After cocaine self-administration training, rats underwent 14 days of forced abstinence in their home cages without exposure to cocaine or cues. This abstinence period was chosen based on prior data demonstrating increased lever pressing in cue-induced reinstatement and more robust DA sensitization following at least 2 weeks of abstinence (Heidbreder et al., 1996; Grimm et al., 2002). On abstinence day 14, rats were returned to the operant chamber for a 2 h cue-induced cocaine-seeking session, where active lever presses led to the CS presentation alone (light + tone) with no cocaine delivery. Inactive lever presses had no programmed consequences. Intra-VTA infusion of pharmacological agents was performed immediately prior to the cue-induced cocaine-seeking test.
Elevated plus maze
The elevated plus maze (EPM) is a plus-shaped maze elevated 70 cm above the floor, that contains a center zone, two open arms, and two enclosed arms. Immediately prior to the 5-minute experiment, all rats were infused with either saline or drug into the VTA to control for the behavioral effects that may be produced by gently restraining the rat for VTA infusion. Following either saline or drug infusion into the VTA, rats were placed in the center of the maze and an overhead video camera and the computer software AnyMaze (Stoelting Company, Wooddale, IL, USA) quantified time spent in center zone, open arms, and closed arms. Number of entries into the open arm and total distance traveled were also recorded. The maze was wiped down with a 70% ethanol solution between test sessions to prevent bias from the scent of the previous rat.
Histological verification
At the completion of all behavioral experiments, rats received a systemic, lethal dose of pentobarbital (150 mg/kg, i.p.) and 0.3 μL of Chicago Blue Dye was infused into the VTA over 2 min via a micro-infusion pump and syringe (25 gauge, Hamilton Syringe, Reno, NE, USA). Rats were then sacrificed, brains were removed, transferred to 3.2% paraformaldehyde for 24 h and then stored in 30% sucrose. Rat brains were placed in a rat brain matrix and sectioned to identify the blue dye placements. 5 rats were excluded from the analysis due to misplacements.
Statistical Analysis
GraphPad Prism 8 (Graph Pad Software, San Diego, CA., USA) and SPSS 24 (IBM, Armonk, New York, USA) were used to conduct statistical analyses. A two-way repeated measures ANOVA was used to analyze cocaine self-administration training data and to verify that groups did not statistically differ in self-administration training behavior, prior to VTA manipulations on test day. The cue-induced cocaine-seeking test data was also analyzed using a two-way repeated measures ANOVA, where lever (active versus inactive) and VTA drug (vehicle versus drug) were the factors. If the two-way ANOVA revealed a significant main effect of drug or lever, or revealed an interaction, a Bonferroni post-hoc analysis was performed to compare between specific drug doses or levers. The within-session active lever presses were analyzed using a two-way repeated measures ANOVA, where dose and time were the factors. If the two-way ANOVA revealed a significant main effect of dose or time, or revealed an interaction, a Bonferroni post-hoc analysis was performed to compare between specific dose and time. The EPM data for cocaine naive rats were analyzed using a one-way ANOVA. If there was a significant main effect, a Bonferroni post hoc analysis was preformed to compare between the groups. For EPM data analysis after cocaine abstinence (Fig. 4), a t-test was used to determine if time spent in the open arms differed between i.v. saline vs. i.v. cocaine groups. For cocaine abstinence EPM experiments, a factorial ANOVA was performed with i.v. drug and VTA drug as factors, and time spent in the open arms as the dependent variable. If the ANOVA revealed a significant main effect, a Bonferroni post-hoc analysis was performed to compare between specific drug groups. Groups were tested for normal distributions and equal variance using the Shaprio-Wilk and Mauchly’s tests. If sampled data were found to violate normal distributions and equal variances, different parametric such as the Brown-Forsythe test were used to account for unequal distributions and variances.
Fig. 4.
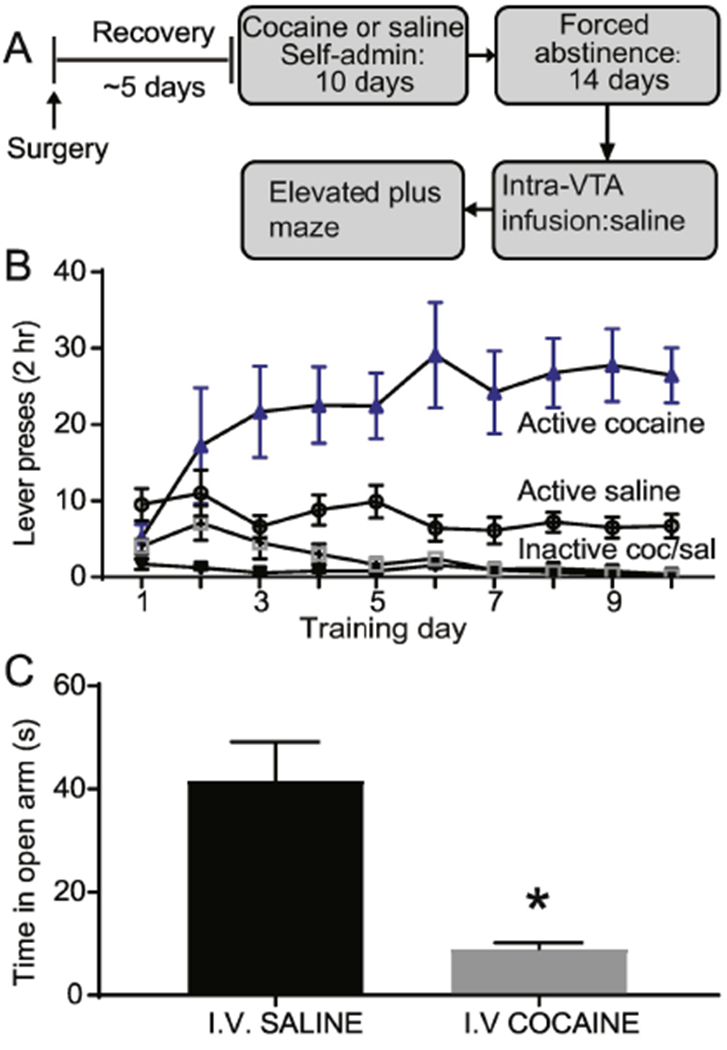
Cocaine self-administration and forced abstinence produces an anxiogenic-like phenotype. (A) Experimental timeline. (B) Self-administration training active vs inactive data for intravenous cocaine and saline rats. (C). Intra-VTA infusion of saline in cocaine abstinence rats showed decreased open arm time in the elevated plus maze, compared to I.V. saline abstinence rats. Error bars indicate the standard error from the mean (SEM). * p < 0.001 (open arm time for saline abstinent vs. cocaine abstinent rats, t-test)
RESULTS
Blockade of VTA muscarinic receptors decreases cue-induced cocaine seeking.
To determine if VTA muscarinic receptor blockade alters cue-induced cocaine-seeking, we first trained rats to self-administer cocaine (~0.5 mg/kg/infusion) (Fig. 1A). Rats readily acquired cocaine self-administration behavior over a 10-day period, as revealed by a main effect of lever (F1, 34 = 113.9, p < 0.01, data not shown) and by a significant lever x day interaction (F9,306 = 7.43, p < 0.001, data not shown). Importantly, rats that would subsequently receive vehicle or scopolamine during the cocaine-seeking test (vehicle or scopolamine) showed no a priori, between group differences in active lever presses (F2, 15 = 0.06, p = 0.95, Fig. 1B), inactive lever presses (F2, 15 = 1.29, p = 0.31, Fig. 1B), or number of cocaine infusions (F2, 15 = 0.19, p = 0.16, data not shown) during self-administration training. On abstinence day 14, VTA infusion of scopolamine significantly reduced cue-induced cocaine-seeking, as shown by a main effect of drug (F2, 15 = 16.84, p < 0.001, Fig. 1C) and by a significant drug x lever interaction (F2, 15 = 29.78, p < 0.001, Fig. 1C). Post-hoc analysis further revealed a significant difference in active lever responding in vehicle versus 2.4 μg scopolamine infused rats (p < 0.001, t-test with a Bonferroni correction, Fig. 1C) and between the vehicle versus 24 μg scopolamine infused rats (p < 0.001, t-test with a Bonferroni correction, Fig. 1C). There was no significant difference in inactive lever responding between vehicle versus 2.4 μg and vehicle versus 24 μg scopolamine (p > 0.99, t-test with a Bonferroni correction, Fig. 1C). On abstinence day 14, VTA infusion of scopolamine significantly reduced active lever responding during the first 30 minutes of the cue-seeking test, as shown by a main effect of drug (F2, 15 = 49.45, p < 0.001, Fig. 1D) and by a significant drug x time interaction (F22, 165 = 4.103, p < 0.001, Fig. 1D). Post-hoc analysis further revealed a significant difference in active lever responding in the first 30 min between vehicle versus 2.4 μg scopolamine infused rats (p < 0.001, t-test with a Bonferroni correction, Fig. 1D) and between the vehicle versus 24 μg scopolamine infused rats (p < 0.001, t-test with a Bonferroni correction, Fig. 1D). VTA placement and injection sites are shown (Fig. 1E). Importantly, prior work from our laboratory has demonstrated that VTA scopolamine infusion does not affect locomotor activity (Solecki et al., 2013; Addy et al., 2015a).
Fig. 1.
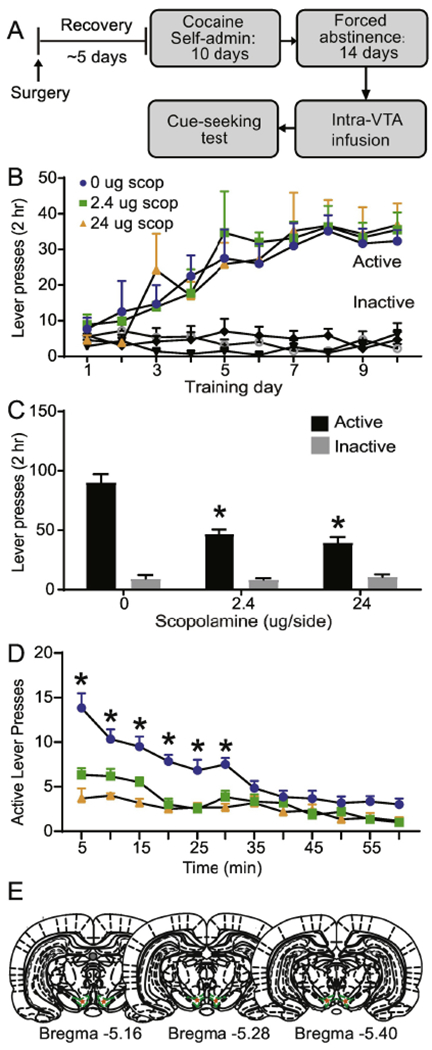
Intra-VTA scopolamine attenuates cue-induced cocaine seeking. (A) Experimental timeline. (B) Cocaine self-administration training showing active vs inactive lever presses, based on experimental assignments for the cue-induced cocaine-seeking test. (C) VTA scopolamine reduces active lever responding but not inactive lever responding. (D) VTA scopolamine attenuates within-session responding on the active lever. (E) Histological verification of VTA infusion placement. Error bars indicate the standard error from the mean (SEM). * p < 0.001 (active lever for 0 vs. 2.4 μg scopolamine and 0 vs. 24 μg scopolamine, post-hoc with Bonferroni correction).
Blockade of VTA nicotinic receptors decreases cue-induced cocaine seeking.
A separate cohort of rats was trained to self-administered cocaine (~0.5 mg/kg/infusion) for ten days (Fig. 2A), and readily acquired the behavior as revealed by a main effect of lever (F1, 42 = 167, p < 0.001, data not shown) and by a significant lever x day interaction (F9,378 = 7.00, p < 0.001, data not shown). Importantly, rats that were vehicle or mecamylamine for the cocaine-seeking test groups showed no a priori, between group differences in active lever presses (F2, 19 = 0.31, p = 0.74, Fig. 2B), inactive lever presses (F2, 19 = 3.10, p = 0.07, Fig. 2B), or number of cocaine infusions (F2,19 = 0.93 , p = 0.41, data not shown) during training. On WD14, VTA infusion of mecamylamine significantly reduced cue-induced cocaine-seeking, as shown by a main effect of drug (F2, 19 = 28.04, p < 0.001, Fig. 2C) and by a significant drug x lever interaction (F2, 19 = 13.63, p < 0.001, Fig. 2C). Post-hoc analysis further revealed a significant difference in active lever responding in vehicle versus 10 μg mecamylamine infused rats (p < 0.001, t-test with a Bonferroni correction, Fig. 2C) and between the vehicle versus 30 μg mecamylamine infused rats (p < 0.001, t-test with a Bonferroni correction, Fig. 2C). There was no significant difference in inactive lever responding between vehicle versus 10 μg and vehicle versus 30 μg mecamylamine (p > 0.99, t-test with a Bonferroni correction, Fig. 2C). On abstinence day 14, VTA infusion of mecamylamine significantly reduced active lever responding during the first 25 minutes of the cue-seeking test, as shown by a main effect of drug (F2, 19 = 21.65, p < 0.001, Fig. 2D) and by a significant drug x time interaction (F22, 209 = 5.734, p < 0.001, Fig. 2D). Post-hoc analysis further revealed a significant difference in active lever responding in the first 25 min between vehicle versus 10 μg mecamylamine infused rats (p < 0.001, t-test with a Bonferroni correction, Fig. 2D) and between the vehicle versus 30 μg mecamylamine infused rats (p < 0.001, t-test with a Bonferroni correction, Fig. 2D). In addition, prior work from our laboratory has demonstrated that these selected doses of mecamylamine do not affect locomotor activity (Solecki et al., 2013; Addy et al., 2015a).
Fig. 2.
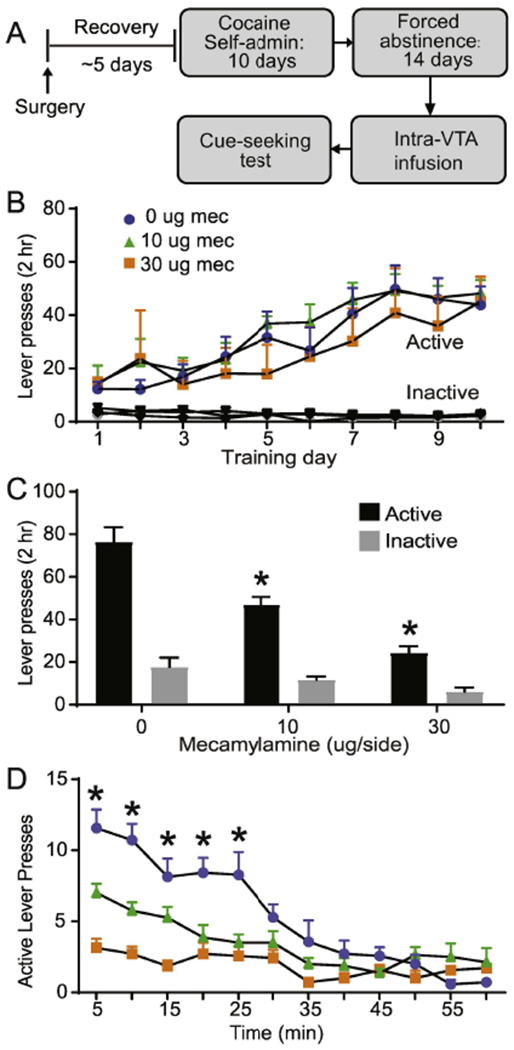
Intra-VTA mecamylamine attenuates cue-induced cocaine seeking. (A) Experimental timeline. (B) Cocaine self-administration training showing active vs inactive lever presses, based on experimental assignments for the cue-induced cocaine-seeking test. (C) VTA mecamylamine reduces active lever responding but not inactive lever pressing. (D) VTA scopolamine attenuates within-session responding on the active lever. Error bars indicate the standard error from the mean (SEM). * p < 0.001, (active lever for 0 vs. 10 μg mecamylamine and 0 vs. 30 μg mecamylamine, post-hoc with Bonferroni correction).
VTA muscarinic and nicotinic receptor blockade in cocaine naive rats produces an anxiolytic-like behavioral phenotype.
In the EPM test, we sought to examine the effects of VTA cholinergic receptor blockade on anxiety-like behavior (Fig. 3A). On test day, VTA infusion of scopolamine significantly increased the time spent in the open arms, as revealed by a significant main effect of drug (F2, 26 = 7.13, p < 0.01, Fig. 3B). Post-hoc analysis further revealed a significant difference between vehicle and 24 μg scopolamine infused rats (p < 0.001, t-test with a Bonferroni correction, Fig. 3B). VTA infusion of scopolamine did not affect number of open arm entries (F2, 26 = 0.28, p > 0.05, data not shown) or total distance traveled (F2,26 = 0.57, p > 0.05, data not shown) between groups. In a separate group of rats, VTA infusion of mecamylamine also significantly increased the time spent in the open arms, as revealed by a significant main effect of drug (F2, 24 = 13.71, p < 0.0001, Fig. 3C). Post-hoc analysis further revealed a significant difference between vehicle treated and 30 μg mecamylamine treated rats (p < 0.0001, t-test with a Bonferroni correction, Fig. 3C). VTA infusion of mecamylamine did not affect number of open arm entries (F2, 24 = 1.30, p > 0.05, data not shown) or total distance travel (F2, 24 = 0.23, p > 0.05, data not shown) between groups.
Fig. 3.
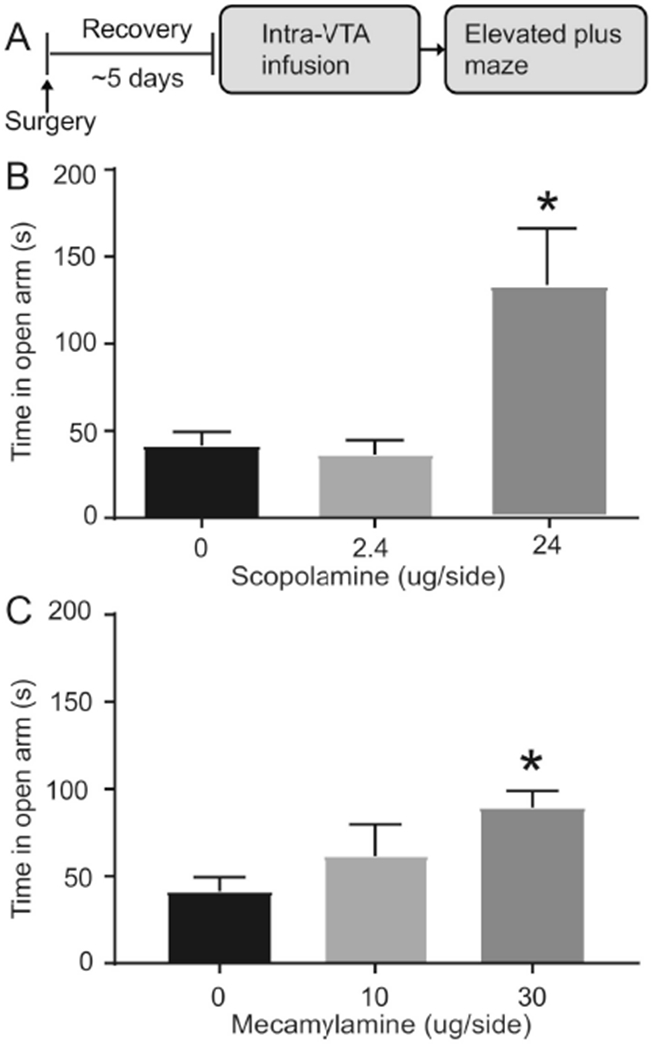
Intra-VTA scopolamine or mecamylamine produces anxiolytic effects in cocaine naive rats. (A) Experimental timeline. (B) VTA scopolamine or (C) VTA mecamylamine infusion led to increased open arm time in the elevated plus maze.. Error bars indicate the standard error from the mean (SEM). * p < 0.05, (open arm time for 0 vs. 2.4 μg scopolamine and 0 vs. 30 μg scopolamine, post-hoc with Bonferroni correction).
Cocaine self-administration and forced abstinence produces an anxiogenic-like phenotype.
A group of rats were trained to self-administer cocaine (~0.5 mg/kg/infusion) or saline for 10 days (Fig. 4A). Cocaine trained rats showed behavioral acquisition, as revealed by a main effect of lever (F1, 80 = 166.6, p < 0.0001, Fig. 4B) and by a significant lever x day interaction (F9,80 = 2.66, p < 0.05, Fig. 4B). Rats that were trained to self-administer saline for 10 days also discriminated between the active and inactive lever, as revealed by a main effect of lever (F1,90 =58.89, p < 0.001, Fig. 4B), but showed no significant lever x day interaction (F9, 90 = 0.61, p < 0.05, Fig. 4B). On abstinence day 14, rats were tested on the EPM. Cocaine abstinent rats spent significantly less time in the open arm compared to saline abstinent rats (t17 = 4.18, p < 0.001, Fig. 4C). Cocaine abstinent rats did not differ on number of open arm entries (t17 = 0.09, p > 0.05, data not shown) or total distance traveled (t17 = 0.08, p > 0.05, data not shown) vs saline abstinent rats.
Blockade of VTA muscarinic receptors reverses the anxiogenic effects of cocaine abstinence.
To determine whether VTA muscarinic receptor blockade would alter the anxiogenic effects of cocaine abstinence, we first trained rats to self-administer cocaine (~0.5 mg/kg/infusion) or saline for 10 days (Fig. 5A). Rats readily acquired the cocaine self-administration behavior, as revealed by a main effect of lever (F1, 19 = 137.8, p < 0.0001, data not shown) and by a significant lever x day interaction (F9,171 = 22.52, p < 0.0001, data not shown). Importantly, rats assigned to vehicle or scopolamine groups on abstinence day 14 showed no a priori, between group differences in active lever presses (F2,17 = 0.31, p = 0.73, Fig. 5B), inactive lever presses (F2,17 = 1.02, p = 0.38, Fig. 5B), or number of cocaine infusions (F2,17 = 0.38, p = 0.69, data not shown) during training. Rats that were trained to self-administer saline for 10 days also discriminated between the active and inactive lever, as revealed by a main effect of lever (F1,50 =35.62, p < 0.0001, Fig. 5B), but showed no significant lever x day interaction (F9, 50 = 0.72, p > 0.05, Fig. 5B) On abstinence day 14, cocaine abstinent rats showed a significant decrease in time spent in the open arms as revealed by a significant main effect of abstinence group (F1,26 = 10.10, p < 0.01, Fig. 5C). There was also a significant effect of VTA drug treatment (F2, 26 = 4.64, p < 0.05, Fig. 5C) on time spent in the open arms in the cocaine abstinent rats. Bonferroni corrected post-hoc analysis further revealed a significant difference between the 2.4 μg and 24 μg versus the 0 μg scopolamine group in the cocaine abstinent rats (p < 0.001), with the 2.4 μg and 24 μg dose spending more time in the open arms compared to 0 μg dose. Furthermore, cocaine abstinent rats that received the 2.4 μg and 24 μg scopolamine showed no difference in open arm time compared to saline abstinence rats (p > 0.05). Number of open arm entries (F2, 26 = 1.08, p > 0.05, data not shown) and total distance traveled (F2, 26 = 0.61, p > 0.05, data not shown) were not significantly different between treatment groups. Together, the data reveal that scopolamine infusion at either the 2.4 μg and 24 μg dose was sufficient to reverse the anxiogenic effect of cocaine abstinence.
Fig. 5.
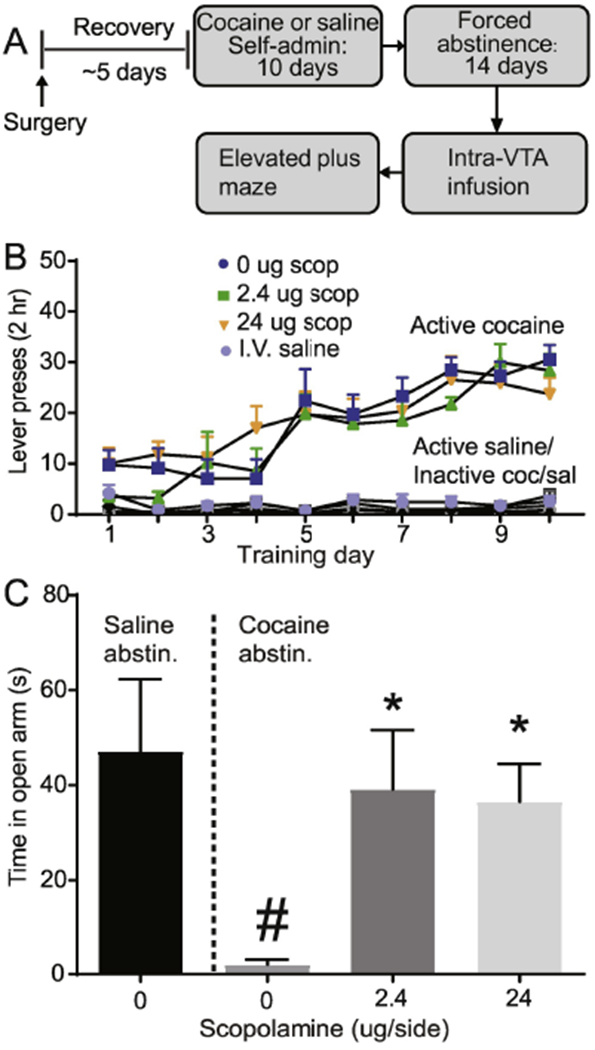
Intra-VTA scopolamine reverses the anxiogenic effects of cocaine abstinence. (A) Experimental timeline. (B) Self-administration training active vs inactive data for intravenous cocaine and saline rats , based on experimental assignments for the subsequent elevated plus maze test. (C) Cocaine abstinence led to decreased open arm time, that was reversed by VTA administration of 2.4 μg or 24 μg scopolamine. (). # p < 0.05 (open arm time in cocaine abstinent vs. saline abstinent rats, factorial ANOVA), * p < 0.05 (open arm time in 0 vs. 2.4 μg scopolamine and 0 vs. 24 μg scopolamine rats).
Blockade of VTA nicotinic receptors reverses the anxiogenic effects of cocaine abstinence.
To determine whether VTA nicotinic receptor blockade also alters the anxiogenic effects of cocaine withdrawal, we trained another cohort of rats to self-administer cocaine (~0.5 mg/kg/infusion) or saline for 10 days (Fig. 6A). Cocaine self-administration behavior was readily acquired as revealed by a main effect of lever (F1, 19 = 147.2, p < 0.0001, data not shown) and by a significant lever x day interaction (F9,171 = 22.38 p < 0.0001, data not shown). Importantly, abstinence day 14 vehicle and mecamylamine rats showed no between group differences in active lever presses (F2,17 = 0.15, p = 0.86, Fig. 6B), inactive lever presses (F2,17 = 1.34, p = 0.28, Fig. 6B), or number of cocaine infusions (F2,17 = 0.07, p = 0.93, data not shown) during training. Rats that were trained to self-administer saline for 10 days also discriminated between the active and inactive lever, as revealed by a main effect of lever (F1,40 =6.90, p < 0.0001, Fig. 6B), but showed no significant lever x day interaction (F9, 40 = 0.72, p > 0.05, Fig. 6B). On abstinence day 14, rats that previously received cocaine showed a significant decrease in time spent in the open arms, as revealed by a significant main effect of abstinence group (F1,25 = 4.80, p < 0.05). There was also a significant effect of VTA drug infusion (F2, 25 = 12.35, p < 0.001, Fig. 6C) on time spent in the open arms in cocaine abstinent rats. Bonferroni corrected post-hoc analysis in cocaine abstinent rats further revealed a significant increase in open arm time in the 10 μg group compared to 0 μg and 30 μg groups (p < 0.001, Fig. 6C), Furthermore, cocaine abstinent rats receiving the 10 μg mecamylamine infusion showed no open arm difference compared to saline abstinent rats (p > 0.05, Fig. 6C). Number of open arm entries (F2, 25 = 2.00, p > 0.05, data not shown) and total distance traveled (F2, 25 = 1.50, p > 0.05, data not shown) was not significantly different between treatment groups Thus, the 10 μg, but not the 30 μg mecamylamine, infusion was sufficient to reverse the cocaine abstinence-induced anxiogenic effect.
Fig. 6.
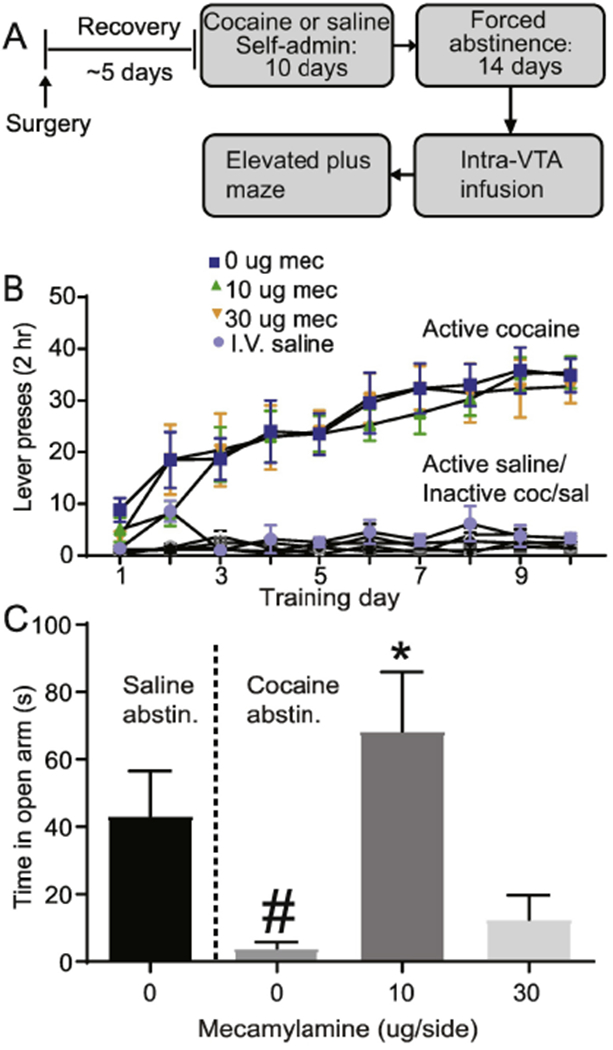
Intra-VTA mecamylamine reverses the anxiogenic effect of cocaine abstinence. (A) Experimental timeline. (B) Self-administration training active vs inactive data for intravenous cocaine and saline rats, based on experimental assignment for the subsequent elevated plus maze test. (C) Cocaine abstinence led to decreased open arm time, that was reversed by VTA administration of 30 μg mecamylamine.. Error bars indicate the standard error from the mean (SEM). # p < 0.05 (open arm time in cocaine abstinent vs. saline abstinent rats, factorial ANOVA), * p < 0.01 (open arm time in 0 vs. 10 μg mecamylamine rats).
DISCUSSION
Here, we examined whether VTA acetylcholine receptors mediate cue-induced cocaine-seeking and anxiety-related behavior following cocaine abstinence. Our data revealed that either VTA mAChR or nAChR blockade with scopolamine or mecamylamine, respectively, robustly attenuated cue-induced cocaine seeking on abstinence day 14. In cocaine naive rats, blockade of VTA muscarinic or nicotinic receptors also produced an anxiolytic-like effect, as reflected by increased open arm time in the EPM test. In contrast, 14-day cocaine abstinent rats showed an anxiogenic-like effect when tested on the EPM, as reflected by decreased open arm time compared to saline abstinent rats. Furthermore, blockade of either VTA mAChRs or nAChRs was sufficient to attenuate the decrease in open arm time observed in of cocaine abstinent rats. Indeed, scopolamine and mecamylamine doses that did not alter EPM behavior in cocaine naive animals were the doses that reversed the cocaine-induced anxiogenic effects. Together, our data reveals an overlapping role for VTA muscarinic and nicotinic receptors in mediating cue-induced cocaine-seeking and an anxiety-related behavior during cocaine abstinence. These data provide support for future investigations to determine the dual role of VTA cholinergic receptors as a therapeutic target to treat cocaine craving and the anxiety-related symptoms during drug abstinence.
Craving and mood-related symptoms in humans are common during abstinence and can facilitate resumed drug use (Shaham and Hope, 2005; Fox et al., 2014; DiGirolamo et al., 2017). Yet, there are no currently approved pharmacotherapies for cocaine craving and the negative affect present during cocaine abstinence. Our current work shows that VTA muscarinic and nicotinic receptor blockade decreased cue-induced cocaine seeking on abstinence day 14, consistent with our previous findings showing the ability of nAChR blockade to decrease cue-induced cocaine-seeking on abstinence day 3 (Solecki et al., 2013). It has also been reported that intra-VTA infusions of muscarinic and nicotinic receptor antagonists, respectively, attenuated the ability of increased VTA cholinergic tone by neostigmine to induce reinstatement of cocaine seeking in rats following extinction training (You et al., 2008). Despite being consistent with our own data, our experimental approach and question were different. In the current study, rats did not receive an extinction trail following cocaine self-administration or a pharmacological manipulation to reinstate cocaine seeking behavior. Rather, our research question focused on the ability of VTA muscarinic and nicotinic receptor blockade to attenuate cue-induced cocaine seeking during forced abstinence. Indeed, emerging literature continues to demonstrate the ability of cholinergic mechanisms (Berizzi et al., 2018) and specifically VTA cholinergic mechanisms (Lof et al., 2007; Zhou et al., 2007; You et al., 2008; Solecki et al., 2013) to alter cue-mediated drug-seeking behaviors. In addition to cue-induced cocaine seeking, VTA mAChR and nAChR blockade also attenuates other components of cocaine reinforcement behavior such as the acquisition and expression of cocaine conditioned place preference (CPP) (Shinohara et al., 2014). It is also possible that cholinergic receptors outside the VTA contribute to cocaine-related behaviors. Indeed, systemic injection of the non-selective nicotinic receptor antagonist mecamylamine during cocaine abstinence prevents the development of cocaine sensitization for a cocaine challenge given after up to 14 days of withdrawal (Schoffelmeer et al., 2002; Szabo et al., 2014). In terms of receptor subtype-specific mAChR effects, genetically altered mice lacking the muscarinic M5 receptor display slower acquisition of cocaine-self administration, spend less time in a cocaine-paired chamber in cocaine CPP, and experience less cocaine-induced withdrawal behaviors (Fink-Jensen et al., 2003). Interestingly, mecamylamine given to human cocaine addicts decreases the ability of cocaine-paired cues to induce feelings of cocaine craving (Reid et al., 1999). Together, our current findings and the previous literature evidence continue to demonstrate the ability of mAChR and nAChR mechanisms to mediate cocaine-related behaviors following days to weeks of abstinence.
Increasing cholinergic tone with the anti-cholinesterase inhibitor physostigmine can induce symptoms of depression and anxiety in healthy humans without substance abuse disorder and those with a prior history of mood-related disorders including unipolar and bipolar depression (Janowsky et al., 1980; Risch et al., 1980; Dulawa and Janowsky, 2018). In preclinical rodent studies, increasing cholinergic tone also produces pro-depressive and anxiogenic responses as measured by the tail suspension test (TST), the forced swim test, the light/dark test, and the elevated plus maze (Mineur et al., 2013; Small et al., 2016; Mineur et al., 2018a). Increasing cholinergic tone specifically in the VTA decreases time spent in the open arms in the EPM, increases immobility time in the FST, and decreases sucrose preference, all consistent with anxiogenic, pro-depressive, and anhedonic-like effects (Addy et al., 2015a; Small et al., 2016). In contrast to VTA AChR activation, our current data demonstrates that blockade of either mAChRs or nAChRs in the VTA produces robust anxiolytic effects in the EPM. In addition, we have previously demonstrated that blockade of VTA mAChRs or nAChRs produces antidepressant-like effects in the FST (Addy et al., 2015a). Together, these findings point to the utility of AChRs as therapeutic targets with potential antidepressant and anxiolytic efficacy. Indeed, systemic administration of scopolamine or mecamylamine has been shown to produce antidepressant-like and anxiolytic-like responses in the TST, FST, and EPM (Andreasen et al., 2009; Andreasen and Redrobe, 2009; Voleti et al., 2013; Wohleb et al., 2016). In humans studies, consistent with the cholinergic hypothesis of depression (Dulawa and Janowsky, 2018) scopolamine has been shown to produce rapid and robust anti-depressant and anxiolytic effects in unipolar and bipolar patients (Furey and Drevets, 2006; Furey et al., 2010). While the preclinical evidence thus far has focused on prefrontal cortex M1 mAChR mechanisms that mediate the rapid antidepressant effects of scopolamine further examinations of midbrain mAChR mechanisms regulating anxiety-related phenotypes are still needed.
Abstinence from cocaine in humans can also produce a variety of adverse mood-related symptoms including depression and anxiety (Gawin, 1991). Our work demonstrates the ability of VTA AChR blockade to not only attenuate cue-induced cocaine seeking but also the robust phenotype that emerges on the EPM during forced abstinence from cocaine as reflected by a reduction of time spent in the open arm. Consistent with our work, others have found that experimenter delivered cocaine followed by abstinence ranging from 24 hours to 4 weeks reduces time spent in the open arms (Sarnyai et al., 1995; Erb et al., 2006; Rudoy and Van Bockstaele, 2007; Perrine et al., 2008; Santucci and Madeira, 2008; de Oliveira Cito Mdo et al., 2012; El Hage et al., 2012). In contrast to experimenter delivered cocaine, EPM experiments after I.V. cocaine self-administration are fewer and with more mixed results. In these experiments, shorter periods of cocaine abstinence have been found to decrease time spent in the open arms on the EPM, while longer periods have had no effect (Buffalari et al., 2012). However, longer abstinent periods from cocaine have been shown to produce an anxiogenic effect on the EPM in the context of a stressor or cue associated with cocaine intake (Erb et al., 2006). In another experiment, extended access to cocaine for 14 days actually increased the amount of time rats spent in time in the open arms, demonstrating and anxiolytic-like phenotype (Mantsch et al., 2008). Despite the mixed results, there are potential reasons why these results have been inconsistent. Factors such as length of exposure to cocaine, extinction training following cocaine self-administration, reinstatement, and exposure to stressors may contribute to the inconsistent findings in the ability of cocaine abstinence to produce an anxiogenic-like effect on the EPM. Nevertheless, our study demonstrates two important findings. First, rats trained to self-administer I.V. cocaine versus I.V. saline for ten days, followed by a forced abstinence period of 14 days displayed a robust anxiogenic-like effect on the EPM. Secondly, VTA nicotinic and muscarinic receptor blockade attenuated both cue-induced cocaine seeking and the anxiogenic-like effect on the EPM during cocaine abstinence.
The nicotinic receptor antagonist, mecamylamine, attenuated both cue-induced cocaine seeking and anxiogenic-like behavior, but at different effective doses for each behavior. Mecamylamine at both doses tested (10 μg and 30 μg) attenuated cue-induced cocaine seeking, while only the low dose (10 μg) reversed the anxiogenic-like response. It is unclear why the high dose (30 μg) was ineffective at increasing open arm time in cocaine exposed rats. However, cocaine use has been shown to effect VTA plasticity and synaptic transmission of DA and GABA neurons (Francis et al., 2019). Moreover, it has been demonstrated that cocaine inhibits and facilitates desensitization of a6 nAChRs expressed on VTA DA neurons (Chen et al., 2019). Therefore, a hypothetical mechanism is that cocaine exposure and abstinence affect plasticity of VTA nAChR subtype expression on DA and GABA neurons. As a result of cocaine-induced plasticity, it is plausible that there is a shift in the dose response curve of the nicotinic receptor antagonist mecamylamine in attenuating the anxiogenic-like phenotype in cocaine abstinent rats. In contrast, the muscarinic receptor antagonist scopolamine attenuated cue-induced cocaine seeking and reversed the anxiogenic-like phenotype at both doses tested (2.4 μg and 24 μg). Thus, it appears that scopolamine is more effective at decreasing both cue-induced cocaine seeking and restoring open arm time across different doses, compared to mecamylamine. However, we have previously found that VTA scopolamine infusion also decreases cue-induced sucrose seeking, whereas mecamylamine infusion does not alter sucrose-seeking (Addy et al., 2015b). Thus, while mecamylamine may have a more limited effective dose range for reversing cocaine’s anxiogenic effects, its attenuation of cue-induced reward-seeking appears to be specific to cocaine (Solecki et al., 2013), without altering cue-induced seeking for a natural sucrose reward (Addy et al., 2015b). Given the effectiveness of VTA mAChR blockade to attenuate cocaine seeking and reverse anxiogenic responses during cocaine abstinence, it would be prudent to investigate receptor-subtype specific effects in future investigations. For instance, it is possible that blockade of a specific mAChR subtype may decrease cocaine seeking and produce anxiolytic effects, without altering sucrose seeking. Furthermore, different subtypes of VTA AChRs may potentially be involved in drug vs. natural cue-induced seeking, and in mediating different mood-related behaviors including anxiety and depression. Indeed, VTA nAChRs are expressed pre- and post-synaptically on GABAergic, glutamatergic and cholinergic terminals, and on DA neurons, all of which can regulate DA activity in the VTA to NAc pathway (Picciotto et al., 1998; Klink et al., 2001; Zoli et al., 2002; Wu et al., 2004; Yan et al., 2018). In particular, the nAChR α6β2 receptor subtype has been shown to regulate DA burst firing (Mameli-Engvall et al., 2006; Exley et al., 2008; Wickham et al., 2013). One potential therapeutic target for further investigation is the M5 mAChR, as either genetic deletion or pharmacological inhibition of M5 has been shown to attenuate cocaine self-administration behavior in rodents (Fink-Jensen et al., 2003; Thomsen et al., 2005; Gunter et al., 2017). Specifically, the M5 mAChR is preferentially expressed in the midbrain, and evidence suggests that M5 expression is localized to post-synaptic VTA DA neurons where M5 mAChRs regulate dopamine release (Vilaro et al., 1990; Weiner et al., 1990; Steidl et al., 2011; Garzon and Pickel, 2013; Foster et al., 2014). As a result, VTA M5 mAChRs may serve as possible therapeutic targets for dopamine-related disorders including addiction, depression, and anxiety.
VTA muscarinic and nicotinic receptors regulate cue-induced cocaine seeking during early abstinence, in part, by regulation of the mesolimbic dopamine system, including the VTA to NAc pathway (Solecki et al., 2013). However, the mechanism by which VTA muscarinic and nicotinic receptors regulate the anxiogenic effects of cocaine self-administration and abstinence are unknown. Yet, work from other labs has also revealed cholinergic receptor dysregulation in other brain areas, including the amygdala and hippocampus, which mediate depression and anxiety-related behavior (Mineur et al., 2016; Mineur et al., 2018b; Mineur et al., 2018a). Nevertheless, we hypothesize that VTA cholinergic receptor regulation of phasic DA release in the VTA to NAc pathway regulates both cocaine-seeking and the anxiogenic-like phenotype during cocaine abstinence by attenuation of phasic DA activity. Indeed, work from several different labs has shown a critical role for the VTA to NAc phasic DA release in both drug-seeking and mood-disorder related behaviors (Phillips et al., 2003; Chaudhury et al., 2013; Tye et al., 2013). Recently, our laboratory has demonstrated that VTA L-type calcium channel blockade with isradipine also attenuates cocaine seeking by actually increasing VTA to NAc phasic DA release (Addy et al., 2018). Therefore, our laboratory has identified two different VTA receptor mechanisms that both decrease cue-induced cocaine seeking during abstinence by either increasing or decreasing phasic DA release in the NAc. Despite the apparent contradiction that increasing DA signaling also attenuates cocaine-seeking, decreases in DA levels have been previously reported during cocaine abstinence (Parsons et al., 1991). This is consistent with the DA depletion hypothesis during cocaine abstinence (Rossetti et al., 1992; Blum et al., 2015). It is possible then that the ability of isradipine to restore DA levels during cocaine abstinence attenuates the cocaine-seeking drive by alleviating the cocaine-induced dopamine depletion. Indeed, systemic injection of either a D1/D2 agonist or the DA precursor L-Dopa is sufficient to decrease cocaine reinstatement in rats (Hasbi et al., 2017; Antinori et al., 2018). Thus, isradipine and other DA replacement drugs may be useful to reduce cocaine relapse by satisfying cocaine craving in human addicts as a result of increased DA levels. Future experiments should examine the ability of DA activating compounds to reduce cocaine craving and anxiety.
One limitation of this study was the use of a single behavioral test to assess a complex construct such as anxiety. Indeed, terms such as anxiogenic and anxiolytic behavior are oversimplifications to describe time spent in either the open or closed arms. Cocaine self-administration followed by periods of abstinence may also be affecting other negative valence systems, which can contribute to an anxiogenic-like effecg on the EPM. It is likely that anxiety overlaps with other behavioral constructs such as acute threat, fear and risk assessment. Therefore, it is plausible that the anxiogenic-like efffect observed during cocaine abstinence is a combination of multiple negative valence constructs. Nevertheless, work from others have demonstrated that anxiogenic-like effects emerge during cocaine abstinence on other behavioral models that measure anxiety such as the light/dark box, open field test, and defensive bury task (Erb et al., 2006; Santucci and Madeira, 2008; Buffalari et al., 2012; de Oliveira Cito Mdo et al., 2012; Georgiou et al., 2016). These findings using other behaviors related to anxiety are consistent with and support the findings in our current study. Nevertheless, future behavioral experiments should incorporate multiple behavioral paradigms that can adequately measure negative valence based behaviors in tandem. Another potential limitation of this study is the ability of impaired VTA cholinergic transmission to produce general deficits in reward-related learning and motor activity (Sharf et al., 2006; Galaj et al., 2017). However, data from our laboratory has demonstrated that these doses of mecamyalmine and scopolamine infused in the VTA does not affect general locomotor activity (Solecki et al., 2013; Addy et al., 2015a) Therefore, our results on both cue-induced cocaine seeking and on the EPM appear not to be related to general behavioral deficits as a result of disrupting VTA cholinergic transmission. Indeed, the ability of VTA cholinergic receptor blockade to increase time spent in the open arms in both cocaine naive and exposed rats indicates that these VTA manipulations are not producing non-specific motor suppressant effects.
In summary, our findings show that cholinergic receptor antagonists injected into the VTA can disrupt relapse and anxiety-related behaviors associated with repeated cocaine administration and abstinence. These results demonstrate the duality of VTA mAChRs and nAChRs in mediating both cue-induced drug seeking, and anxiogenic-like behavior during cocaine abstinence. Moreover, we propose that VTA cholinergic receptor blockade likely disrupts cocaine-mediated plasticity that normally facilitates cue-induced cocaine seeking and anxiety during periods of abstinence. These data provide support for future investigations to determine role of VTA mAChRs and nAChRs as a therapeutic target to treat both cocaine craving and anxiety associated with cocaine exposure and abstinence.
Highlights.
VTA mAChR and nAChR blockade attenuates cue-induced cocaine seeking on forced abstinence day 14.
VTA mAChR and nAChR blockade produces an anxiolytic-like effect on the elevated plus maze in cocaine naive rats.
I.V. cocaine self-administration and forced abstinence produces an anxiogenic-like effect on the elevated plus maze.
VTA mAChR and nAChR blockade attenuates the cocaine-induced anxiogenic-like effect on the elevated plus maze.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Addy NA, Nunes EJ, Wickham RJ (2015a) Ventral tegmental area cholinergic mechanisms mediate behavioral responses in the forced swim test. Behav Brain Res 288:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addy NA, Nunes EJ, Wickham RJ (2015b) Muscarinic, but not nicotinic, acetylcholine receptor blockade in the ventral tegmental area attenuates cue-induced sucrose-seeking. Behav Brain Res 291:372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addy NA, Nunes EJ, Hughley SM, Small KM, Baracz SJ, Haight JL, Rajadhyaksha AM (2018) The L-type calcium channel blocker, isradipine, attenuates cue-induced cocaine-seeking by enhancing dopaminergic activity in the ventral tegmental area to nucleus accumbens pathway. Neuropsychopharmacology 43:2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen JT, Redrobe JP (2009) Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav Pharmacol 20:286–295. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Olsen GM, Wiborg O, Redrobe JP (2009) Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J Psychopharmacol 23:797–804. [DOI] [PubMed] [Google Scholar]
- Antinori S, Fattore L, Saba P, Fratta W, Gessa GL, Devoto P (2018) Levodopa prevents the reinstatement of cocaine self-administration in rats via potentiation of dopamine release in the medial prefrontal cortex. Addiction biology 23:556–568. [DOI] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT (2010) Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend 106:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berizzi AE, Perry CJ, Shackleford DM, Lindsley CW, Jones CK, Chen NA, Sexton PM, Christopoulos A, Langmead CJ, Lawrence AJ (2018) Muscarinic M5 receptors modulate ethanol seeking in rats. Neuropsychopharmacology 43:1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Thanos PK, Oscar-Berman M, Febo M, Baron D, Badgaiyan RD, Gardner E, Demetrovics Z, Fahlke C, Haberstick BC, Dushaj K, Gold MS (2015) Dopamine in the Brain: Hypothesizing Surfeit or Deficit Links to Reward and Addiction. J Reward Defic Syndr 1:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, See RE (2012) Treatment of cocaine withdrawal anxiety with guanfacine: relationships to cocaine intake and reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 223:179–190. [DOI] [PubMed] [Google Scholar]
- Chaudhury D et al. (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493:532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Gao F, Ma X, Eaton JB, Huang Y, Gao M, Chang Y, Ma Z, Der-Ghazarian T, Neisewander J, Whiteaker P, Wu J, Su Q (2019) Cocaine Directly Inhibits alpha6-Containing Nicotinic Acetylcholine Receptors in Human SH-EP1 Cells and Mouse VTA DA Neurons. Frontiers in pharmacology 10:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF (2006) Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 67:247–257. [DOI] [PubMed] [Google Scholar]
- de Oliveira Cito Mdo C, da Silva FC, Silva MI, Moura BA, Macedo DS, Woods DJ, Fonteles MM, de Vasconcelos SM, de Sousa FC (2012) Reversal of cocaine withdrawal-induced anxiety by ondansetron, buspirone and propranolol. Behav Brain Res 231:116–123. [DOI] [PubMed] [Google Scholar]
- DiGirolamo GJ, Gonzalez G, Smelson D, Guevremont N, Andre MI, Patnaik PO, Zaniewski ZR (2017) Increased Depression and Anxiety Symptoms are Associated with More Breakdowns in Cognitive Control to Cocaine Cues in Veterans with Cocaine Use Disorder. J Dual Diagn 13:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Janowsky DS (2018) Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage C, Rappeneau V, Etievant A, Morel AL, Scarna H, Zimmer L, Berod A (2012) Enhanced anxiety observed in cocaine withdrawn rats is associated with altered reactivity of the dorsomedial prefrontal cortex. PloS one 7:e43535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Kayyali H, Romero K (2006) A study of the lasting effects of cocaine pre-exposure on anxiety-like behaviors under baseline conditions and in response to central injections of corticotropin-releasing factor. Pharmacol Biochem Behav 85:206–213. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ (2008) Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology 33:2158–2166. [DOI] [PubMed] [Google Scholar]
- Fatseas M, Serre F, Swendsen J, Auriacombe M (2018) Effects of anxiety and mood disorders on craving and substance use among patients with substance use disorder: An ecological momentary assessment study. Drug Alcohol Depend 187:242–248. [DOI] [PubMed] [Google Scholar]
- Fattore L, Diana M (2016) Drug addiction: An affective-cognitive disorder in need of a cure. Neurosci Biobehav Rev 65:341–361. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Chandler LD, Hutchison KE (2010) Exploring the relationship between depressive and anxiety symptoms and neuronal response to alcohol cues. Alcohol Clin Exp Res 34:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink-Jensen A, Fedorova I, Wortwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile A (2003) Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J Neurosci Res 74:91–96. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Gentry PR, Lizardi-Ortiz JE, Bridges TM, Wood MR, Niswender CM, Sulzer D, Lindsley CW, Xiang Z, Conn PJ (2014) M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor’s location. J Neurosci 34:3253–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Morgan PT, Sinha R (2014) Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology 39:1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Gantz SC, Moussawi K, Bonci A (2019) Synaptic and intrinsic plasticity in the ventral tegmental area after chronic cocaine. Curr Opin Neurobiol 54:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, Han MH (2014) Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Drevets WC (2006) Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry 63:1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Khanna A, Hoffman EM, Drevets WC (2010) Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology 35:2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, Nisanov R, Ranaldi R (2017) Blockade of muscarinic acetylcholine receptors in the ventral tegmental area blocks the acquisition of reward-related learning. Behav Brain Res 329:20–25. [DOI] [PubMed] [Google Scholar]
- Garzon M, Pickel VM (2013) Somatodendritic targeting of M5 muscarinic receptor in the rat ventral tegmental area: implications for mesolimbic dopamine transmission. The Journal of comparative neurology 521:2927–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH (1991) Cocaine addiction: psychology and neurophysiology. Science 251:1580–1586. [DOI] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Hourani S, Kitchen I, Bailey A (2016) Cocaine abstinence induces emotional impairment and brain region-specific upregulation of the oxytocin receptor binding. The European journal of neuroscience 44:2446–2454. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT (2002) Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol 13:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter BW, Gould RW, Bubser M, McGowan KM, Lindsley CW, Jones CK (2017) Selective inhibition of M5 muscarinic acetylcholine receptors attenuates cocaine self-administration in rats. Addiction biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Perreault ML, Shen MYF, Fan T, Nguyen T, Alijaniaram M, Banasikowski TJ, Grace AA, O’Dowd BF, Fletcher PJ, George SR (2017) Activation of Dopamine D1-D2 Receptor Complex Attenuates Cocaine Reward and Reinstatement of Cocaine-Seeking through Inhibition of DARPP-32, ERK, and DeltaFosB. Frontiers in pharmacology 8:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Thompson AC, Shippenberg TS (1996) Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. The Journal of pharmacology and experimental therapeutics 278:490–502. [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Risch C, Parker D, Huey L, Judd L (1980) Increased vulnerability to cholinergic stimulation in affective-disorder patients [proceedings]. Psychopharmacology bulletin 16:29–31. [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21:1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lof E, Olausson P, deBejczy A, Stomberg R, McIntosh JM, Taylor JR, Soderpalm B (2007) Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology (Berl) 195:333–343. [DOI] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, Faure P (2006) Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron 50:911–921. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP (2008) Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 195:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Mose TN, Blakeman S, Picciotto MR (2018a) Hippocampal alpha7 nicotinic ACh receptors contribute to modulation of depression-like behaviour in C57BL/6J mice. Br J Pharmacol 175:1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Fote GM, Blakeman S, Cahuzac EL, Newbold SA, Picciotto MR (2016) Multiple Nicotinic Acetylcholine Receptor Subtypes in the Mouse Amygdala Regulate Affective Behaviors and Response to Social Stress. Neuropsychopharmacology 41:1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR (2013) Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proceedings of the National Academy of Sciences of the United States of America 110:3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Cahuzac EL, Mose TN, Bentham MP, Plantenga ME, Thompson DC, Picciotto MR (2018b) Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety- and depression-related behaviors in mice. Neuropsychopharmacology 43:2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB Jr. (1991) Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse 9:60–65. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates, 6th Edition. London: Elsevier. [Google Scholar]
- Perrine SA, Miller JS, Unterwald EM (2008) Cocaine regulates protein kinase B and glycogen synthase kinase-3 activity in selective regions of rat brain. J Neurochem 107:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM (2003) Subsecond dopamine release promotes cocaine seeking. Nature 422:614–618. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP (1999) A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology 20:297–307. [DOI] [PubMed] [Google Scholar]
- Risch SC, Cohen RM, Janowsky DS, Kalin NH, Murphy DL (1980) Mood and behavioral effects of physostigmine on humans are accompanied by elevations in plasma beta-endorphin and cortisol. Science 209:1545–1546. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Melis F, Carboni S, Gessa GL (1992) Dramatic depletion of mesolimbic extracellular dopamine after withdrawal from morphine, alcohol or cocaine: a common neurochemical substrate for drug dependence. Annals of the New York Academy of Sciences 654:513–516. [DOI] [PubMed] [Google Scholar]
- Rudoy CA, Van Bockstaele EJ (2007) Betaxolol, a selective beta(1)-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Progress in neuro-psychopharmacology & biological psychiatry 31:1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci AC, Madeira E (2008) Anxiogenesis in adult rats treated chronically with cocaine duringadolescence: effects of extended abstinence and 8-OH-DPAT treatment. Brain research bulletin 76:402–411. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G (1995) Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain research 675:89–97. [DOI] [PubMed] [Google Scholar]
- Schellekens AF, de Jong CA, Buitelaar JK, Verkes RJ (2015) Co-morbid anxiety disorders predict early relapse after inpatient alcohol treatment. Eur Psychiatry 30:128–136. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Famous KR, Pierce RC (2009) The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. The European journal of neuroscience 30:1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ (2002) Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci 22:3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Hope BT (2005) The role of neuroadaptations in relapse to drug seeking. Nat Neurosci 8:1437–1439. [DOI] [PubMed] [Google Scholar]
- Sharf R, McKelvey J, Ranaldi R (2006) Blockade of muscarinic acetylcholine receptors in the ventral tegmental area prevents acquisition of food-rewarded operant responding in rats. Psychopharmacology (Berl) 186:113–121. [DOI] [PubMed] [Google Scholar]
- Shinohara F, Kihara Y, Ide S, Minami M, Kaneda K (2014) Critical role of cholinergic transmission from the laterodorsal tegmental nucleus to the ventral tegmental area in cocaine-induced place preference. Neuropharmacology 79:573–579. [DOI] [PubMed] [Google Scholar]
- Sinha R (2013) The clinical neurobiology of drug craving. Curr Opin Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M (2011) Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 218:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KM, Nunes E, Hughley S, Addy NA (2016) Ventral tegmental area muscarinic receptors modulate depression and anxiety-related behaviors in rats. Neurosci Lett 616:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki W, Wickham RJ, Behrens S, Wang J, Zwerling B, Mason GF, Addy NA (2013) Differential role of ventral tegmental area acetylcholine and N-methyl-D-aspartate receptors in cocaine-seeking. Neuropharmacology 75:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl S, Miller AD, Blaha CD, Yeomans JS (2011) M(5) muscarinic receptors mediate striatal dopamine activation by ventral tegmental morphine and pedunculopontine stimulation in mice. PLoS One 6:e27538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo ST, Fowler JC, Froeliger B, Lee TH (2014) Time-dependent changes in nicotine behavioral responsivity during early withdrawal from chronic cocaine administration and attenuation of cocaine sensitization by mecamylamine. Behav Brain Res 262:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB (2005) Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci 25:8141–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K (2013) Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaro MT, Palacios JM, Mengod G (1990) Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci Lett 114:154–159. [DOI] [PubMed] [Google Scholar]
- Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, Sanacora G, Eid T, Aghajanian G, Duman RS (2013) Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry 74:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C (2006) Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26:6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR (1990) Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A 87:7050–7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham R, Solecki W, Rathbun L, McIntosh JM, Addy NA (2013) Ventral tegmental area alpha6beta2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core. Psychopharmacology (Berl) 229:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, Duman RS (2016) GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest 126:2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, George AA, Schroeder KM, Xu L, Marxer-Miller S, Lucero L, Lukas RJ (2004) Electrophysiological, pharmacological, and molecular evidence for alpha7-nicotinic acetylcholine receptors in rat midbrain dopamine neurons. The Journal of pharmacology and experimental therapeutics 311:80–91. [DOI] [PubMed] [Google Scholar]
- Yan Y, Peng C, Arvin MC, Jin XT, Kim VJ, Ramsey MD, Wang Y, Banala S, Wokosin DL, McIntosh JM, Lavis LD, Drenan RM (2018) Nicotinic Cholinergic Receptors in VTA Glutamate Neurons Modulate Excitatory Transmission. Cell reports 23:2236–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Wise RA (2008) Acetylcholine release in the mesocorticolimbic dopamine system during cocaine seeking: conditioned and unconditioned contributions to reward and motivation. J Neurosci 28:9021–9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Liu H, Zhang F, Tang S, Zhu H, Lai M, Kalivas PW (2007) Role of acetylcholine transmission in nucleus accumbens and ventral tegmental area in heroin-seeking induced by conditioned cues. Neuroscience 144:1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C (2002) Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci 22:8785–8789. [DOI] [PMC free article] [PubMed] [Google Scholar]


