Figure 1:
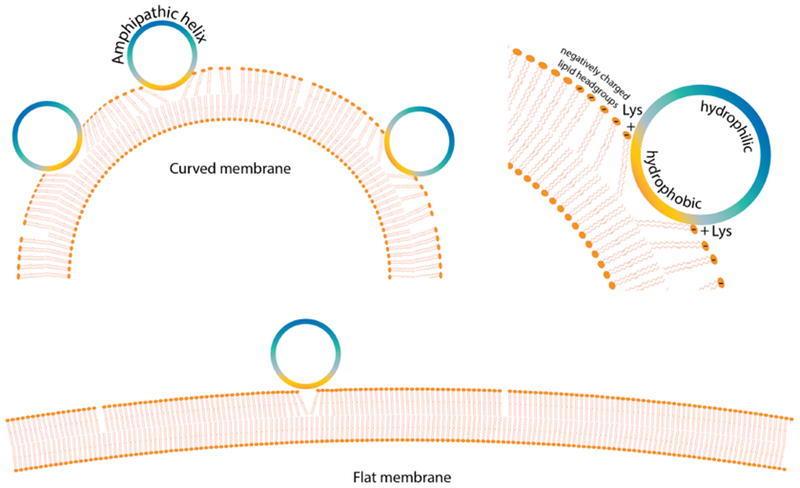
Membrane targeting of IDPs by curvature sensing and electrostatics. Shown here is the example of alpha-synuclein forming an amphipathic helix on preferential binding to highly curved membranes. The outer surface of a highly curved bilayer membrane has a high density of packing defects that allow increased binding and deeper insertion of the hydrophobic face of an amphipathic helix. In the case of alpha-synuclein, such deeper insertion in a negatively charged highly curved membrane is further stabilized by electrostatic interactions between the lysines on the interface and the negatively charged lipid headgroups on the membrane. An imperfectly developed hydrophobic face containing multiple polar threonine residues hinders alpha-synuclein binding on a flat membrane where the packing defects are sparse and shallow, resulting in its curvature sensitivity and preferential binding to negatively charged membranes [55].
