Figure 2:
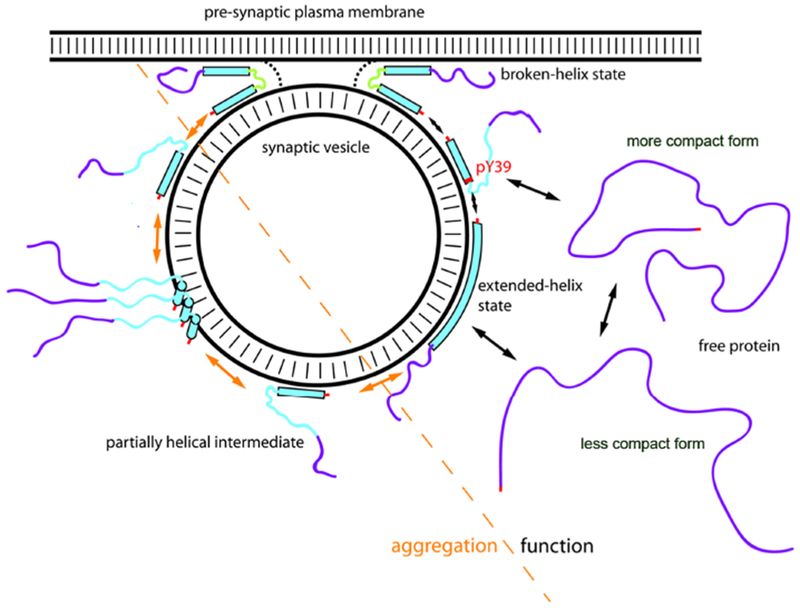
Model for membrane-induced structural changes of alpha-synuclein and their relevance to physiological and disease states. Alpha-helical and intrinsically disordered regions are depicted as filled bars and narrow lines respectively. The membrane-binding domain (residues 1-100) is colored cyan and C-terminal domain (residues 101-140) is colored purple as a visual guide. PTMs including N-terminal acetylation at the N-terminus and phosphorylation at residue tyrosine 39 is indicated are indicated in red. Alpha-synuclein exists in an equilibrium between a disordered monomer in the cytosol that interconverts rapidly between different conformations (although alternative folded oligomeric conformations have been proposed) and multiple membrane-bound conformations on the surface of synaptic vesicles. Upon binding to isolated vesicles, the lipid-binding domain of alpha-synuclein can adopt a highly-extended helical conformation dubbed the extended-helix state, while the C-terminus remains highly disordered, and this interaction is of relatively low affinity in vitro and in vivo. The broken-helix state of the protein, consisting of two shorter helices joined by a non-helical linker, has been observed when alpha-synuclein binds to micelles or to some lipid vesicle compositions, but in this model proposed to allow the protein to bridge between the vesicle and plasma membranes. The broken-helix state binds tightly to micelles in vitro, and membrane-attached synaptic vesicles provide a tight-binding mode for alpha-synuclein in situ as well. In the membrane-bridging mode, the protein is ideally positioned to influence the fusion of the synaptic vesicle with the plasma membrane via a variety of potential mechanisms, including directly modulating the membrane fusion process, or indirect effects mediated by interactions of alpha-synuclein with other proteins that regulate vesicle fusion. The transition to the broken-helix state could be facilitated by phosphorylation of Y39, situated at the beginning of the linker region, which decreases binding of the NAC-containing helix-2 region to isolated vesicles. As a side-effect, phosphorylation of Y39 results in increased population of a partly helical intermediate, similar to that observed in some Parkinson’s disease associated mutants. Such partly helical intermediates are aggregation-prone due to an increased probability of intermolecular interaction of the non-helical and therefore unprotected NAC region and may be the starting point for toxic oligomer and fibril formation in Parkinson’s Disease.
