Abstract
Background: We encountered the opportunity to study proteochemically a brackish water invertebrate animal, Mytilopsis leucophaeata, belonging to the bivalves which stem from the second half of the Cambrian Period (about 510 million years ago). This way, we were able to compare it with the vertebrate animal, the frilled shark (Chlamydoselachus anguineus) that stems from a much later period of geologic time (Permian: 245–286 MYA).
Results: The mussel contains a well-adapted system of protein synthesis on the ER, protein folding on the ER, protein trafficking via COPI or clathrin-coated vesicles from endoplasmic reticulum (ER) to Golgi and plasmalemma, an equally well-developed system of actin filaments that with myosin forms the transport system for vesicular proteins and tubulin, which is also involved in ATP-driven vesicular protein transport via microtubules or transport of chromosomes in mitosis and meiosis. A few of the systems that we could not detect in M. leucophaeata in comparison with C. anguineus are the synaptic vesicle cycle components as synaptobrevin, cellubrevin (v-snare) and synaptosomal associated protein 25-A (t-snare), although one component: Ras-related protein (O-Rab1) could be involved in synaptic vesicle traffic. Another component that we did not find in M. leucophaeata was Rab11 that is involved in the tubulovesicular recycling process of H+/K+-ATPase in C. anguineus. We have not been able to trace the H+/K+-ATPase of M. leucophaeata, but Na+/K+-ATPase was present. Furthermore, we have studied the increase of percent protein expression between 1,070 MYA (the generation of the Amoeba Dictyostelium discoideum) and present (the generation of the mammal Sus scrofa = wild boar). In this time span, three proteomic uprises did occur: 600 to 500 MYA, 47.5 to 4.75 MYA, and 1.4 to 0 MYA. The first uprise covers the generation of bivalves, the second covers gold fish, chicken, brine shrimp, house mouse, rabbit, Japanese medaka and Rattus norvegicus, and the third covers cow, chimpanzee, Homo sapiens, dog, goat, Puccinia graminis and wild boar. We hypothesise that the latter two uprises are related to geological and climate changes and their compensation in protein function expression.
Conclusions: The proteomic and evolutionary data demonstrate that M. leucophaeata is a highly educatioanal animal to study.
Keywords: Mytilopsis leucophaeata, Proteomics, Localisation, Function and adaptation periods
BACKGROUND
Mytilopsis leucophaeata or the brackish water mussel, belonging to the Dreissenidae or bivalve mussels, origi- nated from Europe more than 60 million years ago (Paleocene, Verween et al. 2010). Subsequently, it disap- peared to Central America and returned to Europe (harbour of Antwerp) in 1835. Since then, it is a stable inhabitant of European brackish waters.
In the period of September 2009 to September 2010, two of my colleagues studied the influence of a number of parameters (depth, temperature, salinity and illumin- ation) on size, growth condition, diet and attachment via development of byssal threads (Grutters and Verhofstad 2010). Bivalves have survived a long history from about 510 million years ago to present. This means that it has been adapted to geologically and climate-changing conditions, which might be reflected in the evolution of their proteome. For this reason, the present study was started in order to see whether the presence of certain proteins might unveil certain metabolic systems in this aquatic animal. Almost simultaneously, an article from Riva et al. (2012) did so in relation to the effect of a pol- lutant (triclosan) on the metabolism of Dreissena poly- morpha with emphasis on gills. In the same year, Fields et al. (2012) and Tomanek et al. (2012) published a study on the effect of temperature and hyposalinity on protein expression in the gills of the Mytilidae Mytilus gallopro- vincialis and Mytilus trossulus. In addition, we were inter- ested in comparing amino acid sequences of our mussel with animals stemming from later periods of life in order to cheque the phylogenetic developments that had taken place meanwhile.
METHODS
Forty individuals of the brackish water mussel, caught from a branch of the North Sea Channel to Amsterdam harbour, were taken by scalpel knives and tweezers from their shells, yielding a total wet weight of 1.8 g, sufficient for further analysis. The body parts were taken up in 5 ml triethanolamine HCl, pH 7.0 in 25% glycerol in the pres- ence of 0.5 mM phenylmethylsulfonyl fluoride (PMSF) to prevent autolysis (Schuurmans Stekhoven et al. 2003). Further procedures, such as Potter-Elvehjem homogenisa- tion; fractionated centrifugation to fractions F1, F2 and F3; delipidation of fractions prior to electrophoresis; electro- phoretic separation of proteins in the fractions; staining and destaining of the gels; determination of the apparent molecular weights of the protein bands on gel; excision of the protein bands; transport to the mass spectrometric analysis laboratory in Leicester; as well as the mass spectrometric analysis itself, is given in full detail in our previous publication (Schuurmans Stekhoven et al. 2010).
Information as to the cellular localisation and function of the analysed proteins stem from handbooks like Biochemistry of Hubert Stryer, Google (Scholar), Pubmed. com, BLAST and UniProtKB/Swiss-Prot Protein Knowl- edgebase and literature referred to therein. The absorption spectrum of the brownish coloured F1 fraction (325 to 750nm) was made with a Zeiss M4QIII spectrophotom- eter at 20- to 50-nm intervals. A 100 μlof the F1 fraction was dissolved in 1 ml 2% SDS, subsequently centrifuged for 5 min at 5,000 rpm in a table top centrifuge, and the supernatant scanned.
RESULTS
Homogenisation and fractional centrifugation
Potter-Elvehjem homogenisation of the mussels required very harsh and frequent pottering, yielding a brownish homogenate. Subsequent centrifugation at 1,200, 9,000, and 100,000 g yielded the F1 to F3 fractions. Total pro- tein (mg) of the fractions amounted to 78.3 for F1, 7.03 forF2 and 6.2 for F3, hence ratio F1:F2; F3 = 12.6:1.13:1.0. This ratio brought about association with the kidney (21.5:11.2:1.0) and colon (19.4:3.9:1.0) of the frilled shark Chlamydoselachus anguineus (Schuurmans Stekhoven et al. 2012) in which particular proteins (L-plastin, moesin, fila- min A and α-actinin) are serving as additional construct in linking filaments (microtubules) to the plasma mem- brane. However, in the mussel case, in particular in rela- tion to the brown colour of F1, and less so of F2, we had to think more in terms of byssal threads, the biopolymers by which mussels attach themselves to their substrate like rocks or even ship walls. The brown colour is based on an aqueous solution of pheomelanin (Napolitano et al. 2008) of which the almost exponential absorption curve (down to 325 nm) fits to our curve of M. leucophaeata F1 (Figure 1). Byssal threads apparently are high MW bio- polymers as F1 did not demonstrate any entrance of pro- tein into the gel. This started only in the lightly brown F2 and came to full expression by the light yellow F3, which demonstrated proteins in the apparent molecular weight range of 14.1 to 240 kDa (Tables 1, 2, 3 and 4). The tables are subdivided into prokaryotic and eucaryotic ribosomal subunits (Table 1), proteins from the ER, Golgi network and plasma membrane (Table 2), proteins of the cytoskel- eton and muscle (Table 3) and cellular vacuoles, vaults, nuclei and mitochondria (Table 4). From all these proteins, the prokaryotic or eucaryotic origin is mentioned as well as the function and cellular localisation as could be found in literature, including data banks. All proteins are accompanied by their accession numbers from [UniProtKB/SwissProt] between square brackets. Confu- sion between capital O and the number zero is excluded since capital O is only present at the first position and number zero in any position from 2 to 6 of the accession series. Translation of the accession data to protein easily occurs by using the programme PubMed (www.ncbi.nlm. nih.gov/pubmed) by choosing the term protein.
Fig. 1.
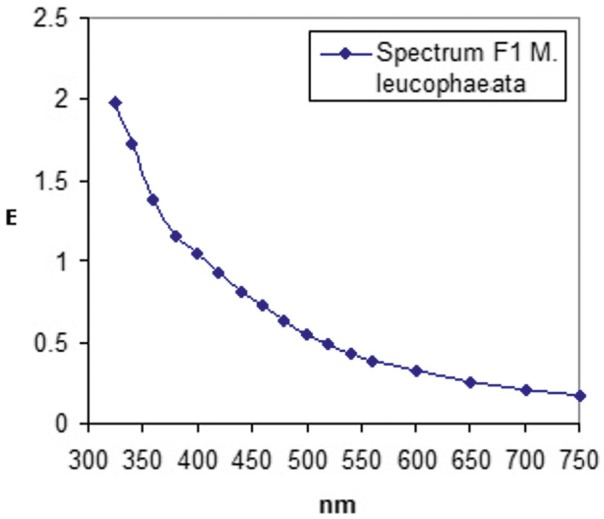
Figure 1 Absorption spectrum of SDS-solubelised F1 fraction from M. leucophaeata from 325–750 nm. Data points are indicated by black spots that have been line connected.
Table1 Ribosomal composition of the F3 fraction (14.1 to 240 kDa) of M.leucophaeata in the prokaryotic and eucaryoticrange.
| Ribosomal subunit | Pro-/eucaryote | Function | Localisation |
| 30S-S1[Q9HZ71] | PseudomonasaeruginosaPAO1 | Proteinsynthesis | Protoplasma |
| 30S-S2[C3K5E6] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 30S-S3[Q3K5Z4] | PseudomonasfluorescensPfO-1 | Idem | Idem |
| 30S-S4[Q3K611] | PseudomonasfluorescensPfO-1 | Idem | Idem |
| 30S-S5[C3K2V9] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 30S-S6[A4XPZ7] | Pseudomonasmendocinaymp | Idem | Idem |
| 30S-S7[C3K2Y0] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 30S-S7[A1AGM8] | Escherichiacoli APECO1 | Idem | Idem |
| 30S-S8[A4VHP4] | PseudomonasstutzeriA1501 | Idem | Idem |
| 30S-S9[C3K6E2] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 30S-S10[A4VHM9] | PseudomonasstutzeriA1501 | Idem | Idem |
| 30S-S11[A4VHQ3] | PseudomonasstutzeriA1501 | Idem | Idem |
| 30S-S13[C3K2V4] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 30S-S14[Q48D49] | Pseudomonassyringaepv.phaseolicola 1448A | Idem | Idem |
| 50S-L1[C3K246] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L1[Q889Y1] | Pseudomonassyringaepv.tomato str. DC3000 | Idem | Idem |
| 50S-L2[C3K2X3] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L3[C3K2X6] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L4[C3K2X5] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L5[C3K2W4] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L6[B1JAJ7] | PseudomonasputidaW619 | Idem | Idem |
| 50S-L9[C3KE70] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L9[A1AJA7] | Escherichiacoli APECO1 | Idem | Idem |
| 50S-L10[C3K2Y5] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L11[C3K2Y7] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L13[C3K6E1] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L14[A4VHP0] | PseudomonasstutzeriA1501 | Idem | Idem |
| 50S-L15[C3K2V7] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L16[C3K2W9] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L17[A4VHQ6] | PseudomonasstutzeriA1501 | Idem | Idem |
| 50S-L18[C3K2W0] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L19[C3K1G8] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L22[A4XZ85] | Pseudomonasmendocinaymp | Idem | Idem |
| 50S-L24[C3K2W5] | PseudomonasfluorescensSBW25 | Idem | Idem |
| 50S-L25[Q3K6W3] | Pseudomonas fluorescensPf0-1 | Idem | Idem |
| 40S-SA[A3RLT6] | Pinctadafucata(pearloyster) | Proteinsynthesis | ER |
| 40S-SA[P38981] | Urechiscaupo (spoonworm) | Assemblyand/or stabilisation ofthe 40S ribosomal subunit | Localisationin adhesion complexes (Willett et al. 2010) |
| 40S-S2[O18789] | Bostaurus (cattle) | Proteinsynthesis | ER |
| 40S-S3[P23396] | Homosapiens (human) | Idem | Idem |
| 40S-S3a[A7S3J7] | Nematostellavectensis (starletsea anemone) | Idem | Idem |
| 40S-S4[Q4GXU6] | Carabusgranulatus(beetle) | Idem | Idem |
| 40S-S6[Q90YR8] | Ictaluruspunctatus (channel catfish) | Idem | Idem |
| 40S-S7[A6H769] | Bostaurus (cow) | rRNAmaturation | Togetherwith NEK6 |
| (serine/threonine kinase) incentrosome (microtubule organising centre, MTOC) | |||
| 40S-S9[A6QLG5] | Bostaurus (cow) | Proteinsynthesis | ER |
| 40S-S13[P49393] | Xenopustropicalis (westernclawed frog) | Idem | Idem |
| 40S-S14[P14130] | Drosophilamelanogaster (fruitfly) | Idem | Idem |
| 40S-S16[P14131] | Musmusculus (housemouse) | Idem | Idem |
| 40S-S17[A5PK63] | Bostaurus (cattle) | Idem | Idem |
| 40S-S18[A5JST6] | Caprahircus (goat) | Idem | Idem |
| 40S-S18 [Q8IT98] | Argopecten irradians (bayscallop) | Idem | Idem |
| 40S-S19[Q94613] | Myaarenaria (soft-shellclam) | Idem | Idem |
| 40S-S24[O42387] | Takifugu rubripes(tigerpuffer) | Idem | Idem |
| 40S-S26[P27085] | Octopusvulgaris | Idem | Idem |
| 40S-S27-like [P24051] | Rattusnorvegicus (Norwayrat) | Idem | Idem |
| 60S-L4[P50878] | Rattusnorvegicus (Norwayrat) | Idem | Idem |
| 60S-L4-B[P02385] | Xenopuslaevis (Africanclawed frog) | Idem | Idem |
| 60S-L5[P09895] | Rattusnorvegicus (Norwayrat) | Idem | Idem |
| 60S-L5[O76190] | Bombyxmori (silkworm) | Idem | Idem |
| 60S-L7a[Q90YW2] | Ictaluruspunctatus (channel catfish) | Idem | Idem |
| 60SL7c[O60143] | Schizosaccharomyces pombe972h- | Idem | Idem |
| 60S-L8[P41569] | Aedesalbopictus (Asiantiger mosquito) | Idem | Idem |
| 60S-L12[E2RR58] | Canislupus familiaris (dog) | Idem | Idem |
| 60S-L16a[P26784] | Saccharomyces cerevisiaeS288c(baker's yeast) | Idem | Idem |
| 60S-L17[A0NGY0] | Anophelesgambiae (Africanmalaria mosquito) | Idem | Idem |
| 60S-L23a[P62750] | Homosapiens (human) | Idem | Idem |
| 60S-L26[P12749] | Rattusnorvegicus (Norwayrat) | Idem | Idem |
Withnames of the animals to whom the proteins are related + function and cellular localisation of these proteins.
Table 2 Proteins from ER, Golgi network and plasma membrane.
| Protein | Eucaryote | Function | Localisation |
| Endoplasmin [O18750] | Oryctolaguscuniculus(rabbit) | Ca2+-binding protein,possibly involved in protein folding (Rowling et al. 1994) | ER |
| Guaninenucleotide-binding protein subunit β[Q5GIS3] | Pinctadafucata(Japanesepearl oyster) | Cargotransport from trans-Golgi network to plasma membrane | Golginetwork and plasmalemma (Irannejadand Wedegaertner 2010) |
| AP-1complex, subunit β-1 [O35643] | Mus musculus (mouse) | Subunitof adaptor protein complex-1, involved in protein sorting, mediating the recruitment of clathrin to the membrane and recognition of sorting signals within the cytoplasmic tails of transmembrane cargo molecules | Trans-Golgi networkand/or clathrin-coated vesicles(UniProt KB/Swiss- Prot:O35643.2, cf. Robinson and Bonifacino 2001) |
| Clathrinheavy chain 1 [P11442] | Rattusnorvegicus | Involved in cargo sorting (cf. adaptor protein complex-1 of Mus musculus) | Clathrin-coated vesicles at the plasma membrane or trans-Golgi network |
| α-Amylase[P04745] | Homo sapiens (human) | Formationof maltose, maltotriose and α-dextrin fromstarch | Inoyster (Crassostreagigas)preferentiallyin digestivetract (Huvet et al. 2003), RER, Golgi, cisternae, condensing vacuoles, and secretory granules (Geuze et al. 1979) |
| Glyceraldehyde-3-phosphate dehydrogenase 2[Q9ESV6] | Rattusnorvegicus | Conversion ofglyceraldehyde-3P to 1,3-bisphosphoglycerate; +Rab2 and protein kinase Ci driven tubulovesicular recycling of proteins from the Golgi to the ER (Tisdale et al. 2009) | Vesiculartubular clusters |
| Transitional endoplasmic reticulum ATPase (TERA) [P03974] | Susscrofa (wildboar) | Involved in fragmentation of Golgi stacks during mitosis and reassembly after mitosis; further is TERA involved in the formation of tER (transitional ER) (UniProt KB/Swiss-Prot information) | Golgi and ER |
| Guanine nucleotide-binding protein G(o), subunit α [O15976] | Mizuhopecten yessoensis (Yesso scallop) | Majorneural signalling GTPase. Reacts on food deprivation (Hofler and Koelle 2011) | |
| IdemG(q), subunit α [P38411] | Lymnaea stagnalis (great pond snail) | ||
| Guanine nucleotide-binding protein subunit β-2-like 1, Rack1 = receptor for activated c kinase-1 [Q93134] | Biomphalaria glabrata (blood fluke planorb = gastropod) | Involved in integrin signalling at adhesions, e.g. in a complex with kindlin-3 | Plasmalemma (Feng et al. 2012) |
| Peptidyl-prolyl cis-trans isomer- ase C (cyclophilin C) [Q08E11] | Bos taurus (cow) | Cyclophilin is a protein folding catalyst | ER (Wang and Heitman 2005) |
| Actin, cytoplasmic 2 (from fibroblastic and epithelial cells) [A2BDB0] | Xenopuslaevis (African clawed frog) | Probablyinvolved in contractile ring formation (Dugina et al. 2009) | Colocalisation withmyosin 2a in stress fibres and with VASP (vasodilator-stimulated phosphoprotein) in lamellipodia and focal adhesions (Dugina et al. 2009) |
| ADP-ribosylation factor1 (Arf 1) [P36579] | Schizosacharomycespombe972h- | Proteintrafficking via COPI or clathrin-coated vesicles from ER to Golgi and plasmalemma | Cis/trans-Golgi andplasmalemma (Chavrierand Goud 1999; D’Souza-Schorey andChavrier 2006) |
| Peptidoglycan-associated lipoprotein[P0A138] | PseudomonasputidaKT2440 | Presence inbivalves may be due to ingestion by the host (Wood 2011) | Cellouter membrane |
| Sarcoplasmic/endoplasmic reticulumcalcium ATPase 3 [Q9YGL9] | Gallusgallus (chicken) | Involvedin muscle contraction | Localisationis in the name |
| Outermembrane porin F [P37726] | Pseudomonasfluorescens | Stabilisationof plasmalemma (multipass membrane protein) | Cellouter membrane (cf. VDAC in C.anguineus, SchuurmansStekhoven et al. 2012) |
| Ribulose bisphosphate carboxylase largechain [A1EA16] | Agrostisstolonifera(creepingbent grass) | Ribulose1,5-bisphosphate + CO2 + H2O →2 3-P-glycerate +2H+, and 3-P-glycerate +2-P-glycolate →ribulose1,5-bisP + O2 | Reactions takeplace in chloroplasts; presence in mollusks indicates contamination by plants |
| Glycogenphosphorylase from liver[P06737] | Homosapiens (human) | Glycogen (n)+ Pi↔glucose-1P +glycogen(n-1) | Microsomal fraction (ER or glycogen particles) (Tata 1964, Margolis et al. 1979) |
| Heatshock 70 kDa protein [P08106] | Gallusgallus (chicken) | Conservationof protein shape and anti-stress protectant (De Maio 1999) | Cytosol, plasma membrane and endosomes +lysosomes (Nylandsted et al. 2004) |
| Heatshock 70 kDa protein cognate 3 [P29844] | Drosophilamelanogaster(fruit fly) | Probablyplays a role in facilitating the assembly of multimeric protein complexes inside the ER | ER(for additional locations and actions see above) |
| 78kDa glucose-regulated protein[Q16956] | Aplysiacalifornica (Californiasea hare =mollusc) | Belongsto the heat shock protein 70 family with function as above | er |
| Dolichyl-diphosphooligosaccharide- protein glycosyltransferase subunit STT3A [P46977] | Homosapiens (human) | Transferof a high mannose oligosaccharide to Asn-X-Ser/Thr in nascent polypeptide chains. The complex associates with Sec61 at the channel forming translocon complex for pro- tein translocation across the ER | ER(expressed at high levels in the placenta, liver, muscle, pancreas; low in the brain, lung, and kidney) |
| Calciumtransporting ATPase [P22700] | Drosophilamelanogaster(fruit fly) | ReversibleCa2+ transport | ViaER and plasmalemma |
| Trypsin[P00761] | Susscrofa(wild boar) | Serineprotease | Indigestive tract (synthesised in pancreas), localised in plasmalemma (Takeuchi et al. 2000) |
| Na+/K+-ATPase, α-subunit [P05025] | Torpedocalifornica (Pacificelectric ray) | 3Na+/2 K+ exchange | Plasmalemma |
| Gelsolin-likeprotein 1 [Q7JQD3] | Lumbricus terrestris (common earth worm) | Regulator ofactin filament assembly and disassembly | Plasmaand intracellular membranes, including ER, cortical vesicles and mitochondria, plus short actin filaments adhering to the plasma membrane (Hartwig et al. 1989) renal brush border membranes |
| Malate dehydrogenase cytoplasmic [Q6PAB3] | Xenopuslaevis (African clawed frog) | Oxidationof malate to oxaloacetate; confers selectivity of thenucleic acid-conducting channel in renal brush border membranes (Hanss et al. 2002) | Renalbrush border membranes |
| Guaninenucleotide- binding protein subunit β-1 [P17343] | Caenorhabditiselegans | Involvedin Gαβγactivationof phospholipase c activity in a cellular signalling process (Yanet al. 2007) | Localisationnot disclosed |
| Ras-relatedprotein O-Rab1 [P22125] | Discopygeommata (ocellated electric ray) | Probablyinvolved in vesicular traffic | O-Rab1 was found largely in the synaptic vesicle fraction (Ngsee et al. 1991) |
| Enolase[O02654] | Loligopealei(longfin inshore squid) | Conversion of 2-phosphoglycerate ↔ phosphenolpyruvate +H2O | Cytoplasmand plasma membrane of synaptosomes (Ueta et al. 2004) |
| 2,3-bisphosphoglycerate- dependent phosphoglycerate mutase[B6IYD3] | RhodospirillumcentenumSW | Catalyses the interconversion of 2-phosphoglycerate and 3-phosphoglycerate | |
| Ras-relatedprotein Rap-1b [A5A6J7] | Pantroglodytes (chimpanzee) | Ca2+ ATPase effector (Lacabaratz-Porret etal. 1998) | Plasmalemma (Marridonneau-Parini andde Gunzburg 1992; Mollinedo et al. 1993) |
Table 3 Proteins of cytoskeleton and muscle.
| Protein | Eucaryote | Function | Localisation |
| Cytoplasmic actin[Q93129] | Branchiostoma Belcheri (Belcher's lancelet) | Transporttrack for myosin + support of cell stability (Pollard and Cooper 2009) | Cytoskeleton |
| Actin,cytoplasmic 1 (β-actin) [P79818] | Oryziaslatipes (Japanese medaka) | Idem | Idem |
| Actin[O16808] | Maietioladestructor (Hessianflyor barley midge) | Cellsupport + providing trafficking routes for myosin in signal transduction (Pollard and Cooper) | cytoskeleton,microfilaments |
| Actin,cytoskeletal 1A [P53472] | Strongylocentrotuspurpuratus (purple sea urchin) | Idem | Idem |
| Actin,non-muscle 6.2 [P17126] | Hydravulgaris(fresh water polyp) | Idem | Idem |
| Actin-3[P53457] | Diphyllobothriumdentricum (flatworm) | Idemin metazoan muscle cells actin forms a scaffold in which myosin generates force to support muscle contraction | Idem |
| Actin-3[P41113] | Podocorynecarnea(jellyfish) | Idem | Idem |
| Actin-18[P07828] | Dictyosteliumdiscoideum (amoeba) | Idem | Idem |
| Actin[O17320] | Crassostreagigas(Pacific oyster) | Idem | Idem |
| Actin[P50138] | Pucciniagraminis(mould) | Idem | Idem |
| Plastin-1[P19179] | Gallusgallus (chicken) | Actinbundling protein | Cytoskeleton |
| Spectrin α-chain, non- erythrocytic 1 [P07751] | Gallusgallus | Playingan important role in membrane organization | Cytoplasm,cytoskeleton and cellcortex |
| Spectrinβ-chain [Q00963] | Drosophila melanogaster (fruit fly) | Spectrinlinks the actin cytoskeleton to the plasma membrane, thus forming a flexible scaffold in the cell cortex (Djinovic-Carugo et al. 2002) | Plasmalemma+ cytoskeleton |
| Heatshock cognate protein HSP90-β [Q04619] | Gallusgallus (chicken) | Earlyembryonic development, germ cell maturation, cytoskeletal stabilisation, cellulartransformation, signal transduction, long-termcell adaptation (Sreedhar et al. 2004) | Cytoplasm+ cytoskeleton (microtubules and actin filaments, Cambiazo et al. 1999) |
| Radixin[P26043] | Musmusculus (mouse) | Participatesin signal transduction and regulatescell migration and intercellular adhesion via Rac 1 (Valderrama et al. 2012) | Linking lasmalemma to actin filaments |
| Ubiquitin[Q86WD4] | Encephalitozooncuniculi (protozoan) | Involvedin the ubiquitin proteasome pathway (Hegde 2010) | Plasmalemmaand cytoskeleton (microtubules) (Murti et al. 1988, Hicke and Dunn 2003) |
| T-complexprotein 1 subunit α[P50157] | Ambystomamexicanum (axolotl, salamander) | TCP-1is chaperonin, involved in protein folding, e.g. of actin and tubulin (Souèset al. 2003; Yam et al. 2008) | Nucleus,cytoskeleton (microtubule organisingcentre) and cytoplasm (Souès et al. 2003) |
| Tubulinβ-chain [P11833] | Paracentrotuslividus (seaurchin) | Mitosis, intracellular vesicle transport (www.buzzle.com/articles/microtubules- function. html) | Partof cytoskeleton: microtubules |
| Tubulinβ-2 chain [P52275] | Caenorhabditiselegans | Partof transport track for ATP-driven vesicle movement or chromosomes in mitosis and meiosis | Idem |
| Tubulinα-1A chain [A5A6J1] | Pantroglodytes(chimpanzee) | Involved insupporting the cell shape and transport of vesicles | Idem |
| Tubulinα-3chain [P05214] | Musmusculus(house mouse) | cf.Tubulin β-chains | |
| Myosin-9 [P35579] | Homo sapiens (human) | Cytokinesis: vesicle transport via actin filaments, cell shape, secretion and capping | Cytoskeleton, cell cortex together with actin filaments at lamellipodia and at the leading edge of migrating cells |
Table 4 Proteins from cellular vacuoles, ribonucleoprotein particles (vaults), nuclei and mitochondria.
| Protein | Eucaryote | Function | Localisation |
| V-typeproton ATPase catalytic subunit A [P314400] | Manducasexta (tobaccohorn worm) | Involvedin cellular trafficking, exocytosis and endocytosis, and interaction with the cytoskeleton (Marshanskyand Futai 2008) | Cellularvacuoles |
| Majorvault protein [Q5EAJ7] | Strongylocentrotuspurpuratus (purple sea urchin) | Signalpathway regulation and immune defence (Berger et al. 2009) | Ribonucleoprotein particles (41× 41 × 71.5 nm) or vaults |
| HistoneH2A.V [P02272] | Gallusgallus (chicken) | Histones play a central role in transcription regulation, DNA repair, DNA replication and chromosomal stability | Partof nucleosome core (nuclei) |
| HistoneH2A [P02268] | Sepiaofficinalis (commoncuttlefish) | Seeabove and below | Seeabove and below |
| HistoneH2B, gonadal [P02284] | Patella granatina (sandpaper limpet) | See above; further, histones and histone fragments circulate via transporters through the cytoplasm and so may be transferred to the plasma membrane (Schuurmans Stekhoven et al. 2004) from where they execute their anti-microbial activity (Seo et al. 2011) | Nuclei,cytoplasm, and plasmalemma |
| Nuclei,cytoplasm, and plasmalemma | |||
| HistoneH3 [P02299] | Drosophilamelanogaster (fruitfly) | Seeabove | Seeover |
| HistoneH4 [P35059] | AcroporaFormosa (stonycorals) | Seeabove | Seeover |
| Proteasome subunitα type-2[Q73672] | Carassiusauratus (goldfish) | Cleavageof peptide bonds with very broad specificity | Cytoplasm andnucleus |
| Proteasome subunitα type-5-A[O81149] | Arabidopsisthaliana (thalecress) | Brakedown of damaged or redundant proteins | Cytosol andnucleus |
| Proteasome subunitα type-7[O13268] | Gallusgallus (chicken) | ATPdependent cleavage of peptide bonds with broadspecificity | Cytoplasm andnucleus |
| 14-3-3protein ε [P92177] | Drosophilamelanogaster (fruitfly) | Multifunctional: regulation ofenzymatic activity, regulation of subcellular localisation, inhibition of protein-protein or protein-DNA interaction, protection against dephosphorylation or proteolytic degradation, stabilisation of multiprotein complexes (Obsiland Obsilova 2011) | Plasmalemma, mitochondrion, nucleus |
| ATPsynthase, subunit α [Q9XXK1] | Caenorhabditiselegans (roundworm) | ATPsynthesis | Mitochondrion |
| ATPsynthase, subunit βprecursor [Q5ZLC5] | Gallusgallus (chicken) | ATPsynthesis | Mitochondrion |
| ATPsynthase, subunit βprecursor [Q5ZLC5] | Xenopustropicalis (Westernclawed frog) | Transferof electrons to coenzyme Q | Mitochondrion |
| ADP/ATPcarrier protein 3 [O49447] | Arabidopsisthaliana (thalecress) | Exchangeof ADP and ATP across the mitochondrial inner membrane | Idem |
| Probablemalate dehydrogenase 3 [Q54VM2] | Dictyosteliumdiscoideum (amoeba) | Oxidationof malate to oxaloacetate | Cytoplasm and mitochondria (Danis and Farkas 2009; Gietl 1992) |
| Phosphoenolpyruvate carboxykinase [Q05893] | Ascarissuum (pigroundworm) | Conversionof oxaloacetate to phosphoenolpyruvate and vice versa | Mitochondria (Stryer1995b) |
Table 1 shows that 52% of the ribosomal subunits is of bacterial origin with a decreasing order in percentage for Pseudomonas fluorescens (32.8%), Pseudomonas stutzeri (7.5%), Pseudomonas mendocina (3%), Pseudomonas syr- ingae (3%), Escherichia coli (3%), Pseudomonas putida (1.5%) and Pseudomonas aeruginosa (1.5%). The other half of the components is occupied by eucaryotes, ran- ging from pearl oyster to Norway rat. Possible causes and impacts of the bacterial contamination will be han- dled under ‘Discussion’ section.
Outside Table 1, only very few bacterial proteins have been identified, except peptidoglycan-associated lipopro- tein in the plasma membrane of P. putida (Table 2), outer membrane porin F from P. fluorescens (Table 2) and 2,3-bisphosphoglycerate-dependent phosphoglycer- ate mutase from Rhodospirillum centenum SW (Table 2). The latter photosynthetic bacterium is housing in mar- ine and brackish water and so can be easily caught by the mussel valves. Still another intruder in the list of proteomics is ribulose bisphosphate carboxylase from Agrostis stolonifera (creeping bent grass) as this reaction takes place in chloroplasts. The habitat of creeping bent grass is on wetlands with tolerance to flooding (Garry Oak Ecosystems Recovery team: www.goert.ca/documents/A.stolonifera.pdf) or inundation of riparian zones which may have brought the plants in contact with the mussels.
Major intracellular activities, presented in Table 2, are protein folding on endoplasmic reticulum (ER) (endoplas- min, peptidyl-prolyl cis-trans isomerase C = cyclophilin C), assembly of multimeric protein complexes inside the ER (heat-shock 70 kDa protein cognate 3, 78 kDa glucose- regulated protein) and protein translocon formation across the ER (dolichyl-diphosphooligosaccharide protein glycosyltransferase). In addition, we found a number of transport processes, such as cargo transport from trans- Golgi to plasma membrane (guanine nucleotide-binding protein), protein sorting at trans-Golgi network and re- cruitment of clathrin to the membrane (AP-1 complex, clathrin heavy chain 1), protein trafficking via COPI or clathrin-coated vesicles from ER to Golgi and plasma- lemma (ADP-ribosilation factor 1 = Arf 1), tubulovesicular recycling of protein from Golgi → ER (glyceraldehyde-3- phosphate dehydrogenase 2) and synaptic vesicle traffic (O- Rab1). Further, a few constructional processes are involved like fragmentation and reassembly of Golgi stacks during and after mitosis + formation of tER (transitional endoplas- mic reticulum ATPase), contractile ring formation (actin, cytoplasmic 2) and regulation of actin filament assembly + disassembly (gelsolin-like protein 1). Subsequently, we rec- ord a number of cellular signalling components like neural signalling GTPase (guanine nucleotide-binding protein G (o) or G(q), subunit α), integrin signalling at adhesions (Rack 1) and Gαβγ activation of phospholipase c in a cellu- lar signalling process (guanine nucleotide-binding pro- tein subunit β-1). Another constructional component is heat-shock 70 kDa protein, yielding conservation of protein shape and protection against stress. In addition, we noticed a number of metabolic enzymes (α-amylase, glycogen phosphorylase, trypsin, enolase) and cation- activated enzymes (sarcoplasmic/endoplasmic reticulum calcium ATPase as modulated by Rap-1b, calcium- transporting ATPase, Na+/K+-ATPase) and the selectivity conferring protein in renal brush border nucleic acid con- ducting channel (cytoplasmic malate dehydrogenase).
The contribution of cellular signalling components ap- pears to be modest in number but has been encountered before in a proteomic analysis of Mytilus galloprovincia- lis and Mytilus trossulus: three to four signalling compo- nents in a total of 47 to 61 proteins, i.e. 6.4% to 6.5% (Tomanek and Zuzow 2010).
The cytoskeletal and muscle components (Table 3) with their numbers between parentheses can be sum- marised as follows: actin non-muscle (11), cytoskeleton (microfilaments) = transport track for myosin; linkers of actin to plasma membrane: plastin, radixin, spectrin α and β chains (4); myosin-9, involved in vesicle transport via actin filaments (1); tubulin α and β chains (microtu- bules, involved in ATP-driven vesicle transport or trans- port of chromosomes in mitosis and meiosis) (4), myosin muscle, involved in contraction: myosin-11 (smooth muscle) (1), myosin LC-1 + heavy chain + adductor muscle light chain (3), paramyosin (byssus retractor muscle) (1), adductor muscle actin (precursor) → contraction (1), actin, muscle precursor, tropomyosin, α-actinin, actin larval muscle (4).
Additional cytoskeletal organised components are: the chaperonin TCP-1, heat-shock cognate protein HSP90-β (with a plurality of functions), ubiquitin (involved in prote- olysis), elongation factor 1α (involved in protein synthesis) and eucaryotic initiation factor 4A-I (involved in mRNA binding to the ribosome).
After corrections for bacterial and plant contamina- tions in Tables 1 and 2, we come to a total of M. leuco- phaeata-related analyses of 112. The analyses, related to the cytoskeletal and muscle components (1st paragraph of Table 3) amount to 30, i.e. 26.8% of all analyses. Simi- lar results have been scored by Tomanek and Zuzow (2010) for M. trossulus and M. galloprovincialis: 16.4% and 25.5%, respectively. In a later study (Fields et al. 2012), they even scored 39.4% and 52.8%, respectively. These numbers underline the importance for these water- bound animals of a sturdy built body with solid protection against predators. Intracellular stability by the cytoskeleton via linking of actin filaments to the cell membrane, pres- ence of adductor muscles for closure of the shell halves provide additional support for the above ideas, and of course, the presence of paramyosin is essential for binding of the animal to the substrate, including stones and ship walls that bring them to the harbours.
In this last part of our analyses (Table 4), we find only transport vesicles (V-type H+-ATPase, major vault pro- tein); histones H2A, H2AV, H2B, H3 and H4; proteasome subunit α type-2, −5A and −7; the 14-3-3 protein ε; ATP synthase subunit α-+ β-precursor; the citric acid cycle en- zymes succinate dehydrogenase; probable malate dehydro- genase 3; and phosphoenolopyruvate carboxykinase that is responsible for the conversion of oxaloacetate to phospho- enolpyruvate in the gluconeogenesis pathway (Stryer 1995b); and the mitochondrial ADP/ATP exchanger.
This gives a fair picture of what is going on in the sub- cellular compartments indicated, but some of the ani- mals (or plants) of comparison (second column) require some criticism with regard to their comparability as will be brought forward in the discussion of Tables 3 and 4.
Fig. 2.
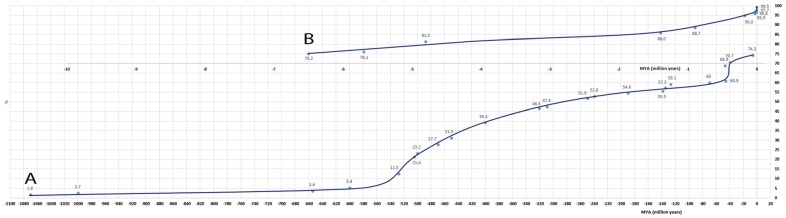
Figure 2 A + B. Graphical expression of pro- and eucaryotes from which proteins were found in the mussel's proteome. The x-axis indicates the time of generation in MYA (million years ago), and the y-axis indicates the accumulated expression in percent of total (100%). Point for point has been connected graphically for the period from 1,070 to 6.5 MYA in part A and from 6.5 to 0 MYA in part B. A complete survey of the data is shown in Table 5.
As mentioned in the ‘Introduction’ section, we were also interested in the protein chemical developments that had taken place in the mussel's long history. For that reason, we have registered the generation time for all vertebrates and invertebrates that had provided se- quences that led to the identification of proteins that have been presented in Tables 1, 2, 3 and 4. Proteins that could be considered as intestinal contaminants: bacterial pro- teins, but also a single plant, living in flooded wetlands, have been omitted. Figure 2A,B provides the graphical re- sult of MYA from 1,100 to zero vs. the sum of the verte- brate + invertebrate contribution. It can be seen that the graph curves upward from 600 to 500 MYA, thereby cov- ering the generation of bivalves. From 320 to 50 MYA, the line is linear, covering the insect Drosophila melanogaster (321 MYA) teleosts Takifugu rubripes and goldfish (70 to 48 MYA). Thereupon follows a second uprise until 4.75 MYA in which period Birds (Phasianidae), Decapoda (brine shrimp), Muridae (rat and house mouse), rabbit and the Japanese medaka participate. After this period, there is some levelling off, but is followed by a third uprise, lasting from 1.4 to 0 MYA (Figure 2B). Animals and fungi, in- volved in this period, are: cow (5), chimpanzee (3), Homo sapiens (7), dog (1), goat (1), stemrust (1) and wild boar (2) (Table 5). From the total of 20 items, 10 (50%) belong to Table 1 and are part of ribosomes that are involved in the protein synthesis machinery. The only cow that was miss- ing in Table 1 can be found in Table 2 as a provider of cyclophilin C, a protein folding catalyst on the ER. Chim- panzee can be found in Table 2 at Ras-related protein Rap- 1b and in Table 3 at tubulin α-1 A chain and eucaryotic initiation factor 4A-I. Rap-1b is a cellular signalling com- ponent in neuronal cells (Sahyoun et al. 1991) and lympho- cytes (Awasthi et al. 2010). Tubulin α-1 A chain is part of the cytoskeleton (microtubules), which supports the cell shape and serves as transport track for vesicles. Eucaryotic initiation factor 4A-I is involved in binding of the messen- ger RNA to the ribosome. H. sapiens (human) is for about half categorised under the ribosomes at Table 1. Further, humans can be found in Table 2 at α-amylase, glycogen phosphorylase, and dolichyl-diphosphooligosaccharide pro- tein glycosyltransferase and in Table 3 at myosin-9. Func- tions are: provision of glucose from its polymers, protein transport across the ER and transport of vesicles along actin filaments. The only dog is related to protein synthesis (Table 1); stemrust (Puccinia graminis) is also related to the cytoskeletal actin (Table 3) and wild boar to transitional ER ATPase (TERA) and the serine protease trypsin (Table 2). The first is involved in breakdown and repair of Golgi stacks during and after mitosis, respectively, plus formation of the transitional ER. The second is involved in proteolysis.
Summarising all processes involved in the third proteo- chemical uprise (covering the Pleistocene and Holocene) shows a composition of protein synthesis, breakdown, folding and transportation across the ER. In addition, binding of messenger RNA to the ribosome, cellular sig- nalling, microtubular vesicle transport, breakdown and repair of Golgi stacks during and after mitosis and for- mation of the transitional ER are also involved. Glucose supply is provided by α-amylase and glycogen phos- phorylase; in the latter case yielding glucose-1P. All these processes are essential for survival of the animal, and during its evolution, it may have been necessary to adapt to the changing environmental conditions.
In addition, we liked to analyse also the second proteo- chemical uprise (48 to 4.75 MYA, corresponding withhalf Eocene to second half of Pliocene). Animals belonging to this time range are chicken (9), brine shrimp (2), house mouse (4), rabbit (1), Japanese medaka (1), and Rattus norvegicus (6), with the number of recorded protein components between parentheses. Summation yields a number of 23, slightly more than the third uprise with 20 components, but taking an appreciably longer time span: 43 vs. 1.4 million years.
Processes involved in this second period ofdevelopment of the mussel are: transcription regulation, DNA repair and replication + chromosome stabilisation (chicken his- tone H2A. V: 1 item), protein synthesis at the ER by ribo- somes from Muridae (Rattus and Mus musculus: 5 items), binding of aminoacyl-tRNA to the ribosomes in protein synthesis (elongation factor 1α, brine shrimp: 1 item),pro- tein folding at the ER (endoplasmin, rabbit: 1 item), cargo sorting at the plasma membrane and trans-Golgi network (clathrin heavy chain 1, R. norvegicus: 1 item, and AP-1 complex, subunit β-1,M.musculus: 1 item), also including endocytosis.
Besides protein synthesis, folding and sorting, there is also the recycling from Golgi to ER (glyceraldehyde-3 phosphate dehydrogenase 2, R. norvegicus: 1 item). Not only protein synthesis is involved, but also protein cleav- age (proteasome subunit α type-7, chicken: 1 item). Pro- tein is transcellularly transported via phosphorylation/ dephosphorylation of myosin on the cytoskeletal actin tracks. Components of this system are provided by brine shrimp (actin, clone 205: 1 item) and Japanese medaka (cytoplasmic actin: 1 item). Intracellular stability is given by linking of actin filaments to each other (plastin-1, chicken: 1 item) or to the plasma membrane (spectrin α chain, chicken: 1 item, and radixin, house mouse: 1 item). Intracellular vesicle transport is provided by tubulin α-3 chain, which is also involved in mitosis (house mouse: 1 item). Heat-shock proteins: 70 kDa and cognate protein HSP 90-β are involved in conservation of protein shape (anti-stress protectant) and cytoskeletal stabilisation + signal transduction (chicken: 2 items). Last, but not least, muscle contraction for closing and opening the valves is effected by sarcoplasmic/endoplasmic reticulum calcium ATPase 3 and myosin-11 (chicken: 2 items). Not to forget is ATP synthase (chicken: 1 item), which will make trans- portation and muscle contraction, besides many other processes, possible via its formation of ATP.
Comparison of the processes, represented by the proteins of the second and third uprise, shows similar- ities and differences. For instance: protein synthesis via ribosomes at the ER counts for 50% in uprise 3, but only for half as much (26%) in uprise 2. Uprise 2 covers transcription regulation, DNA repair and replication + chromosome stabilisation (1 item), whereas uprise 3 does not. Protein folding at ER (1 item) occurs in both periods, but cargo sorting at the plasma membrane and trans-Golgi network (2 items) is only in period 2. The same holds for recycling from Golgi → ER (1 item), whereas protein transport across the ER (1 item) belongs only to period 3. On the other hand, protein cleavage (either by proteasome subunit α type-7 or trypsin) occurs in both periods. The same holds true for actin tracks (2 and 1 item, respectively), but not for actin linkers (3 in period 2 only). Both periods contain vesicle transport (1 and 2 items in periods 2 and 3, respect- ively). Further activities that relate only to period 2 are pro- tein shape conservation + cytoskeletal stabilisation + signal transduction by heat-shock proteins (2 items). Activities that are related to closing and opening the valves, plus the enzyme that this facilitates (ATP synthase) (3 items), only occur in the second uprise, but cellular signalling by Rab-1b and enzymes involved in the hydrolysis of polysaccharides are confined to uprise 3 (4 items).
DISCUSSION
Table 1 showed an abundance of 52% in bacterial riboso- mal subunits. Although the presence of bacteria in the intestine of a eucaryote is a common phenomenon, the capacity of the present bacteria to break down and thereby detoxicate organic pollutants raises the possibil- ity that these bacteria have been added on purpose to the canal inhabited by the mussel. The following detoxify- ing properties have been ascribed to some of the indicated strains: P. fluorescens is beneficial for plants in terms of suppressing pathogens, aiding nutrient absorption and degrading environmental pollutants (www.buzzle.com/articles/pseudomonas-fluorescens.html). P. putida is a versatile environmental isolate that is capable of growth on several aromatic hydrocarbons, including benzene, toluene, ethylbenzene and p-cymene. Its broad substrate toluene dioxygenase has been widely utilised in biocata- lytic synthesis of chiral chemicals, as well as in the me- tabolism and detoxification of trichloroethylene (TCE).
P. putida F1 is known to be chemotactic to aromatic hy- drocarbons and chlorinated aliphatic compounds and has the potential for use in biomediation applications (genome.jgi-psf.org/psepu/psepu.home.html) (site of DOE Joint Genome Institute, University of California). On the other hand, the strain W619 that showed up in our ana- lyses is more competent with regard to heavy metal resis- tances and beneficial effects on plants (Wu et al. 2011).
P. mendocina DSWY0601 and ymp extrude a polyhy- droxybutyrate (PHB) depolymerase that can degrade PHB plastic (Yan et al. 2012). P. stutzeri strain A1501 is equally beneficial to plants by denitrification of NO3−, converting it to N2 and fixation of N2 → 2 NH3. Subse- quently, NH4+ is coupled to α-ketoglutarate under forma- tion of glutamate (Stryer 1995c; Lalucat et al. 2006).
In contrast to the above positive descriptions, the list of bacteria also contains some negatively acting contributors: P. aeruginosa, P. syringae and E. coli. P. aeruginosa, des- pite its positive contribution in oil degradation in the presence of glycerol or the biosurfactant rhamnolipid (Zhang et al. 2005b), also excretes toxins that are dele- terious for the pulmonary system (Roy-Burman et al. 2001). P. syringae is a plant-pathogenic bacterium, in- fecting bean to tomato, causing bacterial speck to bac- terial cancer (P. syringae Genome Resources home page: Pseudomonas-Plant Interaction (PPI) from Cornell University: Department of Plant Pathology: www. pseudomonas-syringae.org). E. coli APEC01 is a dele- terious avian pathogenic bacterium causing epidemic colibacillosis in the poultry industry (Kabir 2010).
Some data of similarity with C. anguineus (Schuurmans Stekhoven et al. 2012) are: synaptic vesicle traffic (Ngsee et al. 1991) and enolase in the plasma membrane of syn- aptosomes (Ueta et al. 2004). The first reminded us of the neurotransmitter cycle that we found in the brain of C. anguineus via its modulator α-synuclein and v- and t- snares VAMP1/2 and SNAP-25 + syntaxin 1. However, although bivalves contain a nervous system (Encyclopaedia Brittanica: www.brittannica.com/EBchecked/topic/67293/ bivalve/35745/The-shell), we have not been able to find the abovementioned v- or t-snares for bivalves. In partial contrast to this are the results obtained for M. galloprovin- cialis (Venier et al. 2009) in which results for three t-snares in the Mediterranean mussel have been obtained via tran- scribed sequences: SNAP-25A [Accession No. Q5TZ66], SNAP-type protein [Accession No. Q25391] and SNAP-47 [Accession No. Q0P4A7]. Yet, v-snares have not been detected either in this case. Presence of enolase in the plasma membrane appears to have an endangering effect via its complex formation with plasminogen that by subsequent activation to plasmin can break down the extracellular matrix and so can allow invasion of patho- gens, viruses and metastatic cancer cells (Liu and Shih 2007; Díaz-Ramos et al. 2012). Normally, plasmin is used to dissolve fibrin blood clots but upon generation on the cell surface might cause the above effects. How- ever, in our analyses, neither plasminogen nor plasmin (MW 81 and 75.4 kDa, Barlow et al. 1969) or plasmino- gen activator (tPA, MW 72 kDa, Manosroi et al. 2001) has been traced. On the other hand, in the transcribed sequences of M. galloprovincialis, two sequences were found that matched plasminogen [Accession No's. Q01177 and Q6PBA6] (Venier et al. 2009). Furthermore, despite the clear presence of Na+/K+-ATPase in our analyses, we have been unable to find the presence of phospholemman (FXYD1), known as a modulator of Na+/K+-ATPase (Mahmmoud et al. 2000), even though our analyses covered a wide range of molecular weights (14.1 to 240 kDa). Since phosphorylation of Na+/K+-ATPase causes dissociation of phospholem- man, this may have led to its absence in the analyses. In addition, salinity may also decrease the FXYD con- tent relative to the Na+/K+-ATPase content (Wang et al. 2008). In another report (Horisberger 2006), it has been indicated that no FXYD protein can be found in arthro- pods or any nonvertebrate animals. We think that the only way that is left to trace the absence or presence of FXYD in bivalves is to analyse their DNA.
One of the fungi that have entered the list of com- parative sequences is P. graminis at actin in Table 3. This mould spreads its occurrence by spore formation via two different hosts, thereby causing the so-called stemrust, especially in wheat and barley (Schumann and Leonard 2000). Although contamination of the brackish water mussel with infected wheat and/or barley from freight ships in the harbour cannot be excluded, another possibility is indistinguishable peptides formed by trypsin treatment (cf. Schuurmans Stekhoven et al. 2010) as used in the analysis of actin from Crassostrea gigas [Accession No. O17320] or Puccinia graminis [Accession No. P50138]. A few of the possibilities are a20gfagddapr29, h41qgvmvgmgqk51 and y70piehgivtnwddmek85 for Crassostrea gigas and the same sequences for Puccinia graminis, but with a numbering of a19-r28, h40-k50 and y69-k84. It appears that the sequences are quite conserved since they are also found in β-actin of the mammal M. musculus [Accession No. ABL01512].
Another subject of criticism is the possibly hereditary plant sequences in the genome of the mussel. An ex- ample could be the occurrence of Proteasome subunit α type-5A and ADP/ATP carrier protein from Arabidopsis thaliana (thale cress). Thale cress grows on edges of agricultural fields, stone walls alongside tracks and roads and Mediterranean scrublands with scattered holm oaks but is no inhabitant of wet lands (Picó et al. 2008). Therefore, we have looked for comparable sequences in MytiBase: a knowledgebase of mussel (M. galloprovincialis) with 3,275 transcribed sequences (Venier et al. 2009). To our surprise: also in this large list of transcribed proteins, a few examples of plant heritage were met: first the occur- rence of 14-3-3-like protein b of Oryza sativa (India Group) [Accession No. ABR25888] together with 14-3-3 C1 protein from Oncorhynchus mykiss [Accession No. Q6UFZ7]. Identical sequences, found for Oryza sativa with those for O. mykiss (the latter between parentheses), are e16-e34 (e113-e131), p65-f80 (p162-f177), l94-d100 (l191-d197) and s113-d134 (s210-d231). It is evident that the sequence for Oryza sativa is 97 amino acids less than that of O. mykiss, due to incomplete DNA. Yet, we can calculate an identity that must be minimally 25% the same. Since the sequences in case of M. galloprovincialis have been determined via DNA, we have to accept a genetic link between animals and plants, and so the link with proteasome subunit αtype 5A, ADP/ATP carrier protein 3 and Arabidopsis thaliana may be genuine and not artificial.
A second example of plant heritage by M. galloprovin- cialis is found in the presence of probable ATPase from the chloroplast of Oenothera organensis (organ Mountains evening primrose) [Accession No. Q0H0T1] which grows in the mountains of New Mexico, far away from the mussel of the Mediterranean Sea. Hence, there has been a time that they were neighbours. This hypothesis is built on three assumptions:
Some DNA ofconsumed food can be taken up in cells and incorporated into the DNA of the consumer if it displays some similarity with DNA of that consumer
Since bivalves do not inhabit the mainland, the plant consumers could be snails (Gastropods), which form a sister clade with bivalves, forming the Pleistomollusca (Kokot et al. 2011).
If geological conditions, like flooding, would force gastropods to evolve to bivalves, it is not unthinkable that bivalves would contain land plant sequences in their genome as has been shown by Venier et al. (2009). Before accepting this hypothesis, it will be necessary to trace the snail's genome or RNA for plant resemblances.
In the generation of the brackish water mussel M. leuco- phaeata in the period of 1,070 to 0 MYA, two additional genetic uprises occurred beyond the uprise caused by the generation of the bivalves per se (approximately 510 MYA). The question arises why this has to be achieved by acceleration in a developmental uprise: the second in the period of 48 to 4.75 MYA and the third in the period of 1.4 to 0 MYA. In the 48 to 4.75 MYA period (Eocene-Pliocene), earth was in motion with formation of mountains and separation or collision of geological plates, volcano formation, followed by climate cooling (Pidwirny 2012). In the later period (1.4 to 0 MYA: Pleistocene + Holocene), earth was subject to freezing (Pleistocene Ice Age) with extinction of many species (Pidwirny 2012), which may have forced the mussel to a counterreaction by speeding up its adaptation of DNA to that of modern species (cow, chimpanzee, human, dog, stemrust and wild boar, Table 5).
Evidence for climate change-induced effects on adap- tation of the genome of animals and plants has been recently published (Reusch and Wood 2007; Buckley et al. 2012; Franks and Hoffmann 2012). Failure to adapt to the changes may eventually lead to extinction. In this respect, M. leucophaeata did not fail, otherwise it could not have been able to survive for 500 million years until present (Figure 2). Therefore, it is remarkable that it has only a limited range of salt concentration: 6.7 to 7.4 ppt (0/00) to provide for an optimal condition. At higher sa- linities, the condition index is reduced to 50 at a salinity of 11 ppt (Grutters and Verhofstad 2010). Seawater usu- ally has a salinity of 35 ppt (Office of Naval Research: www.onr.navy.mil/focus/ocean/water/salinity 1.htm). There- fore, one may question how M. leucophaeata can survive the trip via the ocean to the brackish North Sea Channel. Transportation of larvae and postlarvae with tolerance to salinity of 32 ppt in ballast water makes the trip possible (Verween et al. 2010). The salinity of the North Sea Channel varies from 1.7 to 9.2 ppt (Van der Velde et al. 1998), which M. leucophaeata can enter with confidence.
Acknowledgments
Acknowledgements
We like to thank M.Sc. Michiel Verhofstad and M.Sc. Bart Grutters for their gift of M. leucophaeata, which is at the basis of this study. As in my previous publication (Schuurmans Stekhoven et al. 2012), M.Sc. Ruud van Hintum helped me by photography of the gels after electrophoresis of the F1 to F3 fractions. Also, K. van Benthem is acknowledged for the construction of Figure 1 of this paper and my son M.G.H.P. Schuurmans Stekhoven for the construction of Figure 2, Lady S. Ibrahim (Protein and Nucleic Acid Chemistry Laboratory, Proteomics Facility), University of Leicester for her contribution in the protein analyses of this paper and of course Kiki Wu for her textual settings.
Footnotes
Authors’ contributions: FSS carried out the homogenisation and fractional centrifugation of the bivalve homogenate, electrophoresis of the fractions, staining and destaining of the gels and conveying the gel strips to the analytical laboratory of ARB in Leicester. Further, he interpreted the data and wrote this article. THL offered his contribution in formatting of the text and provided his data (included in this paper) on the effect of salinity change on the expression of FXYD, a modulator of Na+/K+-ATPase. GvdV, the malacologist of our department, provided essential information on the anatomy and physiology of mollusks, and ARB, together with Lady S. Ibrahim, analysed the gel strips and sent me the proteomic data together with their accession numbers in the data banks. All authors read and approved the final manuscript.
Competing interests: The authors declare that they have no competing interests.
References
- Awasthi A, Samarakoon A, Chu H, Kamalakannan R, Quiliam L A, Chrzanowskawodnicka M, White G C, Malarkannan S. Rap-1b facilitates NK cell functions via IQGAP1-mediated signalosomes. J Exp Med. 207:1923–1938. doi: 10.1084/jem.20100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley N. Cambrian Period: Facts & Information. 2013.
- Barlow G H, Summaria L, Robbins K C. Molecular weight studies on human plasminogen and plasmin at the microgram level. J Biol Chem. 244:1138–1141. [PubMed] [Google Scholar]
- Baxeranis A D, Kappas I, Abatzopoulos T J. Molecular phylogenetics and asexuality in the brine shrimp Artemia. Mol Phyl Evol. 40:724–738. doi: 10.1016/j.ympev.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Berger W, Steiner E, Grusch M, Elbling L, Micksche M. Vaults and the major vault protein: novel roles in signal pathway regulation and immunity. Cell Mol Life Sci. 66:43–61. doi: 10.1007/s00018-008-8364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco M, Ferrand N, Monnerot M. Phylogeography of the European rabbit (Orectolagus cuniculus) in the Iberian Peninsula inferred from RFLP analysis of the cytochrome b gene. Heredity. 85:307–317. doi: 10.1046/j.1365-2540.2000.00756.x. [DOI] [PubMed] [Google Scholar]
- Buckley J, Butlin R K, Bridle J R. Evidence for evolutionary change associated with the recent range expansion of the British butterfly, Aricia agestis, in response to climate change. Mol Ecol. 21:267–280. doi: 10.1111/j.1365-294X.2011.05388.x. [DOI] [PubMed] [Google Scholar]
- Cambiazo V, González M, Isamit C, Maceoni R B. The β-isoform of heat shock protein hsp-90 is structurally related with human microtubule-interacting protein Mip-90. FEBS Lett. 457:343–347. doi: 10.1016/s0014-5793(99)01070-4. [DOI] [PubMed] [Google Scholar]
- Cartwright P, Halgedahl S L, Hendricks J R, Jarrad R D, Marques A C, Collins A G, Lieberman B S. Exceptionally preserved jellyfishes from the Middle Cambrian. PLoS One; 2007. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Goud B. The role of ARF and Rab GTPases in membrane transport. Curr Opin Cell Biol. 11:466–475. doi: 10.1016/S0955-0674(99)80067-2. [DOI] [PubMed] [Google Scholar]
- Souza-Schorey D', Chavrier C. ARF proteins: roles in membrane traffic and beyond. Nature Rev Cell Biol. 7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- Danis P, Farkas R. Hormone-dependent and hormone-independent control of metabolic and developmental functions of malate dehydrogenase. Review Endocrine Reg. 43:39–52. doi: 10.4149/endo_2009_01_39. [DOI] [PubMed] [Google Scholar]
- De Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 11:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- Díaz-Ramos À, Roig-Borellas A, Garcia-Melero A. López-Alemany R (2012) α-Enolase a multifunctional protein: its role on pathophysiological situations. J Biomed Biotech; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djinovic-Carugo K, Gautel M, Ylänne J, Young P. The spectrin repeat: a structural platform for cytoskeletal assemblies. FEBS Lett. 513:119–123. doi: 10.1016/s0014-5793(01)03304-x. [DOI] [PubMed] [Google Scholar]
- Dugina V, Zwaenepoel I, Gabbiani G, Clément S, Chaponnier C. ) β-and γ-cytoplasmic actins display distinct distribution and functional diversity. J Cell Sci. 122:2980–2988. doi: 10.1242/jcs.041970. [DOI] [PubMed] [Google Scholar]
- Feng C, Li Y-F, Yan Y-H, Lee H-S, Tang X-Y, Xue Z-H, Zhou Y-C, Lim W-M, Cornvik T C, Ruedl C, Schochat S G, Tan S-M. Kindlin-3 mediates integrin αLβ2 outside-in signaling, and it interacts with scaffold protein receptor for activated-c kinase 1 (Rack1) J Biol Chem. 287:10714–10726. doi: 10.1074/jbc.M111.299594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P A, Zuzow M J, Tomanek L. Proteomic responses of blue mussel (Mytilus) congeners to temperature acclimation. J Exp Biol. 215:1106–1116. doi: 10.1242/jeb.062273. [DOI] [PubMed] [Google Scholar]
- Franks S J, Hoffmann A A. Genetics of climate change adaptation. Ann Rev Genet. 46:185–208. doi: 10.1146/annurev-genet-110711-155511. [DOI] [PubMed] [Google Scholar]
- Furano A V, Usdin K. DNA "fossils" and phylogenetic analysis, using L1 (Line-1, long interspersed repeated) DNA to determine the evolutionary history of mammals. J Biol Chem. 270:25301–25304. doi: 10.1074/jbc.270.43.25301. [DOI] [PubMed] [Google Scholar]
- Germonpré M, Sablin M V, Stevens R E, Hedges Rem, Hofreiter M, Stiller M, Després V R. Fossil dogs and wolves from Paleolithic sites in Belgium, the Ukraine and Russia: ostometry, ancient DNA and stable isotopes. J Archaeol Sci. 36:473–490. [Google Scholar]
- Geuze J J, Slot J W, Tokuyasu K T, Goedemans Wem, Griffith J M. Immunochemical localization of amylase and chymotrypsinogen in the exocrine pancreatic cell with special attention to the Golgi complex. J Cell Biol. 82:697–707. doi: 10.1083/jcb.82.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietl C. Malate dehydrogenase isoenzymes: cellular locations and role in the flow of metabolites between the cytoplasm and cell organelles. Biochim Biophys Acta. 1100:217–234. doi: 10.1016/0167-4838(92)90476-t. [DOI] [PubMed] [Google Scholar]
- Grimaldi D, Engel M S. Evolution of the insects, Part of Cambridge Evolution Series. Cambridge): Cambridge University Press; 2005. [Google Scholar]
- Grutters Bmc, Verhofstad Mjjm. Assessing and comparing three invasive bivalves: Dreissena polymorpha, Dreissena rostriformis bugensis and Mytilopsis leucophaeata. Nijmegen): 2010. [Google Scholar]
- Hanss B, Leal-Pinto E, Teixeira A, Christian R E, Shabanowitz J, Hunt D F, Klotman P E. Cytosolic malate dehydrogenase confers selectivity of the nucleic acid conducting channel. Proc Natl Acad Sci U S A. 99:1707–1712. doi: 10.1073/pnas.022355499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardjasasmita H S. Taxonomy and phylogeny of the Suidae (Mammalia) in Indonesia. Scripta Geol. 85:1–68. [Google Scholar]
- Hartwig J H, Chambers K A, Stossel T P. Association of gelsolin with actin filaments and cell membranes of macrophages and platelets. J Cell Biol. 108:467–479. doi: 10.1083/jcb.108.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges S B. The origin and evolution of model organisms. Nat Rev Genet. 3:838–849. doi: 10.1038/nrg929. [DOI] [PubMed] [Google Scholar]
- Hegde A N. The ubiquitin-proteasome pathway and synaptic plasticity. Learn Mem. 17:314–327. doi: 10.1101/lm.1504010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding protein. Ann Rev Cell Dev Biol. 19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Hofler C, Koelle M R. AGS-3 alters Caenorhabditis elegans behavior after food deprivation via Ric-8 activation of the neural G protein Gα o. J Neurosci. 31:11553–11562. doi: 10.1523/JNEUROSCI.2072-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger J-D. Evolution of Na-K-ATPases. Plenary Lecture LT_1.02. 99, Annual Meeting of the German Zoological Society; 1920. [Google Scholar]
- Abstractband Münster.
- Huvet A, Daniel J-Y, Quéré C, Dubois S, Van Wormhoudt Prudence M, Sellos A, Samain D, Moal J-F. Tissue expression of two α-amylase genes in the pacific oyster Crassostrea gigas: Effects of two different food rations. Aquaculture. 228:321–333. [Google Scholar]
- Irannejad R, Wedegaertner P B. Regulation of constitutive cargo transport from the trans-Golgi network to plasma membrane by Golgi-localized G protein βγ subunits. J Biol Chem. 285:32393–32404. doi: 10.1074/jbc.M110.154963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir Sml. Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int J Environ Res Public Health. 7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas Geological Survey (2005) www.kgs.ku.edu/Extension/fossils/gastropod.html. 2005.
- Kansas Geological Survey (2008) www.kgs.ku.edu/Extension/fossils/bivalve.html. 2008.
- Kislev ME. Stem rust of wheat 3300 years old found in Israel. Science. 1982;216:993–994. doi: 10.1126/science.216.4549.993. [DOI] [PubMed] [Google Scholar]
- Kokot K M, Cannon J T, Todt C, Citarella M R, Kohn A B, Meyer A, Santos S R, Schander C, Moroz L L, Lieb B, Halanych K M. Phylogenomics reveals deep molluscan relationships. 477:452–457. doi: 10.1038/nature10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacabaratz-Porret C, Corvazier E, Kovàcs T, Bobe R, Bredoux R, Launay S, Papp B, Enouf J. Platelet sarco/endoplasmatic reticulum Ca 2+ ATPase isoform 3b and rap 1b: interaction and regulation in physiopathology. Biochem J. 332:173–181. doi: 10.1042/bj3320173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalucat J, Bennasar A, Bosch R, Garcia-Valdés E, Palleroni N J. Biology of Pseudomonas stutzeri. Microbiol Mol Biol Rev. 70:510–547. doi: 10.1128/MMBR.00047-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K-J, Shih N-Y. The role of enolase in tissue invasion and metastasis of pathogens and tumor cells. J Cancer Mol. 3:45–48. [Google Scholar]
- Liu G, Tang J, Edmonds B T, Murray J, Levin S, Condeels J. F-actin sequesters elongation factor 1α from interaction with aminoacyl-tRNA in a pH-dependent reaction. J Cell Biol. 135:953–963. doi: 10.1083/jcb.135.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugh Mac, Shriver D E, Loftus M D, Cunningham R T, Bradley P. Microsatellite DNA variation and the evolution, domestication and phylogeography of Taurine and Zebu cattle (Bos taurus and Bos indicus) Genetics. 146:1071–1086. doi: 10.1093/genetics/146.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmmoud Y A, Vorum H, Cornelius F. Identification of a phospholemman-like protein from shark rectal glands. Evidence for indirect regulation of Na, K-ATPase by protein kinase C via a novel member of the FXYD family. J Biol Chem. 275:35969–35977. doi: 10.1074/jbc.M005168200. [DOI] [PubMed] [Google Scholar]
- Manosroi J, Tayapiwatana C, Götz F, Werner R G, Manosroi A. Secretion of active recombinant human tissue plasminogen activator derivatives in Escherichia coli. Appl Environ Microbiol. 67:2657–2664. doi: 10.1128/AEM.67.6.2657-2664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R N, Cardell R R, Curnow R T. Association of glycogen synthase phosphatase and phosphorylase phosphatase activities with membranes of hepatic smooth endoplasmic reticulum. J Cell Biol. 83:348–356. doi: 10.1083/jcb.83.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marridonneau-Parini I, De Gunzburg J. Association of rap1 and rap2 proteins with the specific granules of human neutrophils: translocation to the plasma membrane during cell activation. J Biol Chem. 267:6396–6402. [PubMed] [Google Scholar]
- Marshansky V, Futai M. The V-type H + -ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol. 20:415–426. doi: 10.1016/j.ceb.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlikovský J. A new guinea fowl (Aves, Phasianidae) from the late Eocene of France. Ann Naturhist Mus Wien. 90:63–66. [Google Scholar]
- Mollinedo F, Perez-Sala D, Gajate C, Jimenez B, Rodriguez P, Lacal J C. Localization of rap1 and rap2 proteins in the gelatinase-containing granules of human neutrophils. FEBS Lett. 326:209–214. doi: 10.1016/0014-5793(93)81792-x. [DOI] [PubMed] [Google Scholar]
- Murti K G, Smith H T, Fried V A. Ubiquitin is a component of the microtubule network. Proc Natl Acad Sci U S A. 85:3019–3023. doi: 10.1073/pnas.85.9.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano A, De Lucia M, Panzella L, Ischia M. The "benzothiazine" chromophore of pheomelanins: a reassessment. Photochem Photobiol. 84:593–599. doi: 10.1111/j.1751-1097.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- Ngsee J K, Elferink L A, Scheller R H. A family of ras-like GTP-binding proteins expressed in electromotor neurons. J Biol Chem. 266:2675–2680. [PubMed] [Google Scholar]
- Nyitray L, Goodwin E B, Szent-Györgyi A G. Complete primary structure of a scallop striated muscle myosin heavy chain. Sequence comparison with other heavy chains reveals regions that might be critical for regulation. J Biol Chem. 266:18469–18476. [PubMed] [Google Scholar]
- Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Høyer-Hansen M, Weber E, Multhoff G, Rohde M, Jäättelä M. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 200:425–435. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obsil T, Obsilova V. Structural basis of 14-3-3 protein functions. Semin. Cell Dev Biol; 2011. [DOI] [PubMed] [Google Scholar]
- Picó F X, Méndez-Vigo B, Martínez-Zapater J M. Natural genetic variation of Arabidopsis thaliana is geographically structured in the Iberian Peninsula. Genetics. 180:1009–1021. doi: 10.1534/genetics.108.089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidwirny M. Geologic time, The Encyclopedia of Earth. Washington D.C): 2012. [Google Scholar]
- Pollard T D, Cooper J A. Actin, a central player in cell shape and movement. Science. 326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharks Pre-Historic. About prehistoric Sharks-Megalodon, fossil teeth. 2012.
- Reid Gbr, Hetherington R. The climate connection: climate change and human evolution. Cambridge, UK): Cambridge University Press; 2010. [Google Scholar]
- Reusch Tbh, Wood T E. Molecular ecology of global change. Mol Ecol. 16:3973–3992. doi: 10.1111/j.1365-294X.2007.03454.x. [DOI] [PubMed] [Google Scholar]
- Riva C, Cristoni S, Binelli A. Effects of triclosan in the freshwater mussel Dreissena polymorpha: a proteomic investigation. Aquatic toxicol. 118:62–71. doi: 10.1016/j.aquatox.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Robinson M S, Bonifacino J S. Adapter-related proteins. Curr Opin Cell Biol. 13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- Rowling Pje, Mclaughlin S H, Pollock G S, Freedman R B. A single purification procedure for the major resident proteins of the ER lumen: endoplasmin, BIP, calreticulin and protein disulfide isomerase. Prot Expr Purif. 5:331–336. doi: 10.1006/prep.1994.1049. [DOI] [PubMed] [Google Scholar]
- Roy-Burman A, Savel R H, Racine S, Swanson B L, Revadigar N S, Fujimoto J, Sawa T, Frank D W, Wiener-Kronish J P. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Inf Dis. 183:1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- Sahyoun N, Mcdonald O B, Farrell F, Lapetina E G. Phosphorylation of Ras-related GTP-binding protein, Rap-1b, by a neuronal Ca 2+ /calmodulindependent protein kinase, CaM kinase Gr. Proc Natl Acad Sci U S A. 88:2643–2647. doi: 10.1073/pnas.88.7.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G L, Leonard K J. Stem Rust of Wheat (black rust). The Plant Health Instructor. 2000.
- Schuurmans Stekhoven Fmah, Grell E, Atsma W, Flik G, Bonga Wendelaar. Organ-related distribution of phospholemman in the spiny dogfish Squalus acanthias. Biochem Biophys Res Commun. 303:1008–1011. doi: 10.1016/s0006-291x(03)00472-8. [DOI] [PubMed] [Google Scholar]
- Schuurmans Stekhoven Fmah, Bonga Wendelaar, Flik S E. Extranuclear histones in teleost gills: an evolutionary study. Fish. Physiol Biochem. 30:201–211. [Google Scholar]
- Schuurmans Stekhoven Fmah, Bonga Wendelaar, Lee S E, Bottrill T-H. Presence or absence of the Cl − channel phospholemman in the rectal gland of sharks: a comparative study. Zool Studies. 49:326–334. [Google Scholar]
- Schuurmans Stekhoven Fmah, Lee T-H, Wang P-J, Jang-Liaw N-H, Tanaka S, Bottrill A R. Proteomic studies of various organs and tissues of the frilled shark Chlamydoselachus anguineus. Zool Studies. 51:1248–1269. [Google Scholar]
- Seo J-K, Stephenson J, Noga E J. Multiple antibacterial histone H2B proteins are expressed in tissues of American oyster. Comp Biochem Physiol B. 158:223–229. doi: 10.1016/j.cbpb.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Smith A B. Allen & Unwin; 1984. 1 [Google Scholar]
- Souès S, Kann M-L, Fouquet J-P, Melki R. The cytosolic chaperonin CCT associates to cytoplasmic microtubular structures during mammalian spermiogenesis and to heterochromatin in germline and somatic cells. Exp Cell Res. 288:363–373. doi: 10.1016/s0014-4827(03)00248-9. [DOI] [PubMed] [Google Scholar]
- Sreedhar A S, Kalmár E, Csermely P, Shen Y-F. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 562:11–15. doi: 10.1016/s0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- Stryer L. The Power Stroke in Contraction is Driven by Conformational Changes in the Myosin S1 Head. WH Freeman and Company. pp. 399–400.
- Stryer L. Phosphoenolpyruvate Carboxykinase, 4th edn. Biochemistry, WH Freeman and Company; 1995. [Google Scholar]
- Stryer L. Nitrogen Fixation: Microorganisms Use ATP and a Powerful Reductant to Reduce N 2 to NH 3 , 4th edn. Biochemistry, WH Freeman and Company. pp. 714–717.
- Takehana Y, Nagai N, Matsuda M, Tsuchiya K, Sakaizumi M. Geographic variation and diversity of the cytochrome b gene in Japanese wild populations of Medaka, Orizias latipes. Zool Sci. 20:1279–1291. doi: 10.2108/zsj.20.1279. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Harris J L, Huang W, Yan K W, Coughlin S R, Craik C S. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 275:26333–26342. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- Tata J R. Subcellular redistribution of liver α-glucan phosphorylase during alterations in glycogen content. Biochem J. 90:284–292. doi: 10.1042/bj0900284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale E J, Azizi F, Artalejo C R. Rab 2 utilizes glyceraldehyde-3-phosphate dehydrogenase and protein kinase Ci to associate with microtubules and to recruit dynein. J Biol Chem. 284:5876–5884. doi: 10.1074/jbc.M807756200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek L, Zuzow M J. The proteomic response of the mussel congeners Mytilus galloprovincialis and M. trossulus to acute heat stress: implications for thermal tolerance limits and metabolic costs of thermal stress. J Exp Biol. 213:3559–3574. doi: 10.1242/jeb.041228. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Zuzow M J, Hitt L, Serafini L, Valenzuela J J. Proteomics of hyposaline stress in blue mussel congeners (genus Mytilus): implications for biogeographic range limits in response to climate change. J Exp Biol; 2012. [DOI] [PubMed] [Google Scholar]
- Ueta H, Nagasawa H, Oyabu-Manabe Y, Toida K, Ishimura K, Hori H. Localisation of enolase in synaptic plasma membrane as an αγ heterodimer in rat brain. Neurosc Res. 48:379–386. doi: 10.1016/j.neures.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Valderrama F, Therapala S, Ridley A J. Radixin regulates cell migration and cell-cell adhesion through Rac 1. J Cell Sci. 125:3310–3319. doi: 10.1242/jcs.094383. [DOI] [PubMed] [Google Scholar]
- Van Der Velde G, Van Der Gaag M, Rajagopal S, Jenner H A. Where do the exotic mussels Dreissena polymorpha and Mytilopsis leucophaeata meet in the brackish Noordzeekanaal. Sacramento, USA): The Netherlands? In: 8th International Zebra Mussel and Other Aquatic Nuisance Species Conference; [Google Scholar]
- Venier P, De Pittà C, Bernante F, Varotto L, De Nardi B, Bovo G, Roch P, Novoa B, Figueras A, Pallavicini A, Lanfranchi G. MytiBase: a knowledge base of mussel (M. galloprovincialis) transcribed sequences. BMC Genomics; 2009. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verneau O, Catzeflis F, Furano A V. Determining and dating recent rodent speciation events by using L1 (Line-1) retrotransposons. Proc Natl Acad Sci U S A. 95:11284–11289. doi: 10.1073/pnas.95.19.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verween A, Vincx M, Degraer S. Mytilopsis leucophaeata: the brackish water equivalent of Dreissena polymorpha? A review. Backhuys Publishers, Leiden/ Margraf Publishers. pp. 29–44.
- Veyrunes F, Dobigny G, Yang F, O'brien Pcm, Catalan J, Robinson T J, Britton-Davidian J. Phylogenomics of the genus Mus (Rodentia, Muridae): extensive genome repatterning is not restricted to the house mouse. Proc R Soc B. 273:2925–2934. doi: 10.1098/rspb.2006.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volff J-N. Genome evolution and biodiversity in teleost fish. Heredity. 94:280–294. doi: 10.1038/sj.hdy.6800635. [DOI] [PubMed] [Google Scholar]
- Waggoner B M. Anthozoa: Fossil Record; 2000. [Google Scholar]
- Wang P, Heitman J. The cyclophilins. Genome Biol; 2005. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Junbing L, Shunping H. Molecular evidence for the monophyly of East Asian groups of Cyprinidae (Teleostei, Cypriniformes) derived from the nuclear recombination activating gene 2 sequences. Mol Phyl Evol. 42:157–170. doi: 10.1016/j.ympev.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Wang P-J, Lin C-H, Hwang H-H, Lee T-H. Branchial FXYD protein expression in response to salinity change and its interaction with Na + /K + -ATPase of the euryhaline teleost Tetraodon nigroviridis. J Exp Biol. 211:3750–3758. doi: 10.1242/jeb.018440. [DOI] [PubMed] [Google Scholar]
- Wegener Parfrey L, Lahr Djg, Knoll A H, Katz L A. Estimating the time of early eukaryotic diversification with multigene molecular clocks. Proceed Natl Acad Sci; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett M, Pollard H J, Vlasak M, Morley S J. Localization of ribosomes and translation initiation factors to talin/β3-integrin-enriched adhesion complexes in spreading and migrating mammalian cells. Biol Cell. 102:265–276. doi: 10.1042/BC20090141. [DOI] [PubMed] [Google Scholar]
- Won Y J, Hey J. Divergence population genetics of chimpanzees. Mol Biol Evol. 22:297–307. doi: 10.1093/molbev/msi017. [DOI] [PubMed] [Google Scholar]
- Wood R R. Analysis of bacterial communities in the eastern oyster (Crassostrea virginica) with emphasis on Vibrio vulnicus dynamics under refrigeration. Alabama): 2011. [Google Scholar]
- Wu X, Monchy S, Taghavi S, Zhu W, Ramos J, Van Der Lelie D. Comparative genomics and functional analysis of niche-specific adaptation in Pseudomonas putida. FEMS Microbiol Rev. 35:299–323. doi: 10.1111/j.1574-6976.2010.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam A Y, Xia Y, Lin H-Tj, Burlingame A, Gerstein M, Frydman J. Defining the TRIC/CCT interactome links chaperonin function to stabilization of newly-made proteins with complex topologies. Nat Struct Mol Biol. 15:1255–1262. doi: 10.1038/nsmb.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Sato M, Lanier S M, Smrcka A V. Signaling by a non-dissociated complex of G protein βγ and α subunits stimulated by a receptor-independent activator of G protein signaling AGS8. J Biol Chem. 282:19938–19947. doi: 10.1074/jbc.M700396200. [DOI] [PubMed] [Google Scholar]
- Yan W, Fan L, Zhan-Yong W, Dong-Bo L, Hong-Mei X, Ling-Fei L, Shan C. Purification and properties of an extracellular polyhydroxybutyrate depolimerase from Pseudomonas mendocina DSWY0601. Chem Res Chin Univ. 28:459–464. [Google Scholar]
- Zhang P, Zhou H, Chen Y-Q, Liu Y-F, Qu L. Mitogenomic perspectives of the origin and phylogeny of living amphibians. Syst Biol. 54:391–400. doi: 10.1080/10635150590945278. [DOI] [PubMed] [Google Scholar]
- Zhang G-L, Wu Y-T, Qian X-P, Meng Q. Biodegradation of crude oil by Pseudomonas aeruginosa in the presence of rhamnolipids. J Zhejiang Univ (Sci) 6:725–730. doi: 10.1631/jzus.2005.B0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M E, Souda P, Jin Y-P, Whitelegge J P, Reed E F. Characterization of the endothelial cell cytoskeleton following HLA class I ligation. Plos ONE; 2012. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]