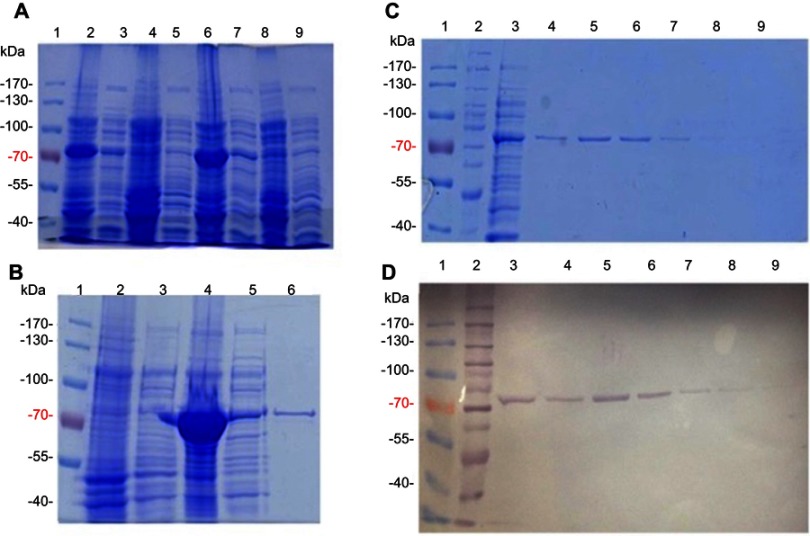
Figure 1.
Purification of E. coli PPK1-His6- tag by immobilized metal affinity chromatography (IMAC). Recombinant E. coli PPK1-His6-tag was purified by immobilized Ni-Sepharose affinity chromatography as previously reported.8 Bacterial Protein Extraction Reagent B-PER® (Thermo Fisher Scientific, Waltham, MA, USA) was used for obtaining the recombinant proteins from the bacterial pellet. (A) Insoluble and soluble fractions of cell proteins respectively from 4 different colonies (lanes 2–9) were obtained by centrifugation and visualized in Tris/Glycine 12% SDS–PAGE stained with Coomassie Blue. (B) Insoluble fraction of selected colony (line 4) was discarded and the respective soluble fraction (line 5) was equilibrated and loaded onto the affinity column (His-Trap ®; GE Healthcare, Chicago, IL, USA) to obtain the purified PPK1-His6-tag protein (line 6). Insoluble and soluble protein extracts from negative expression control were loaded into lines 2 and 3. (C, D) Fractions were eluted with 200 mM de imidazole and were analyzed by Tris/Glycine 12% SDS–PAGE. Non-retained fraction (line 3) and eluted fraction (lines 4–9) were stained with Coomassie Blue (C) and also by Western-Blot (D) using Nickel-HRP, HisDetectorTM kit (KPL Inc., Milford, MA, USA).