Fig. 4. LPXTG-adhesin C-peps interact with SGO_1180.
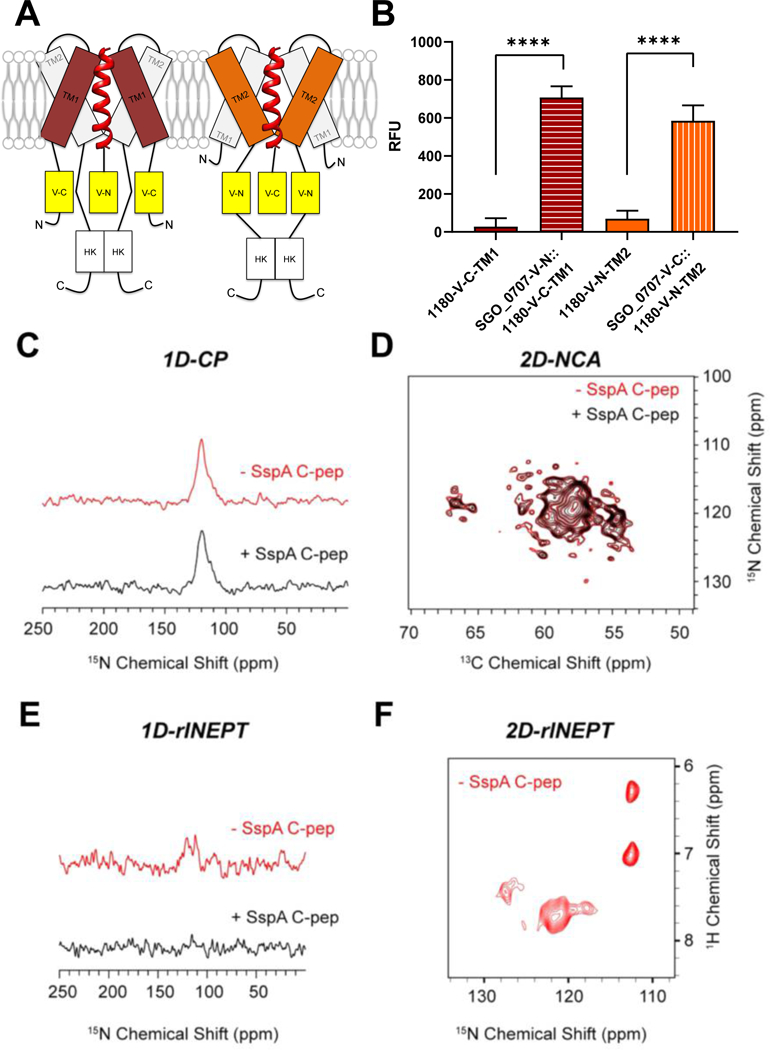
(A) Schematic of the interaction between SGO_0707 C-pep (red helical ribbon) and transmembrane domains (TM1 and TM2) of SGO_1180. The N- or C-terminal (N or C) domain of Venus YFP was inserted in-frame between the penultimate and stop codon of sgo_0707 and on the cytoplasmic side of TM1 or TM2 of sgo_1180. All four combinations of the constructs that could reconstitute active YFP were tested in cells, but only the two that produced fluorescence are shown in the diagram. (B) Quantification of fluorescence emission from the single and dual-labeled bifluorescent strains shown in (A). Fluorescence was only detected when the N-terminal domain of YFP fused to the SGO_0707 C-pep (SGO_0707-V-N) was combined with the C-terminal domain of YFP fused to TM1 of SGO_1180 (1180-V-C-TM1) or when the C-terminal domain of YFP fused to the SGO_0707 C-pep (SGO_0707-V-C) was combined with the N-terminal domain of YFP fused to TM2 of SGO_1180 (1180-V-C-TM2). RFU, relative fluorescence units, fluorescence units divided by the culture optical density at λ = 600 nm. Data represents the mean ± SD of three independent experiments with three technical replicates per experiment. Data were analyzed using an unpaired Student’s t-test. Significant differences between strains are denoted by asterisk(s): * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p ≤ 0.0001. (C-F) Magic-angle spinning (MAS) NMR spectroscopy of the SspA C-pep and SGO_1180 (1:1) in artificial lipid membranes. The 1D cross-polarization (CP) spectrum to detect the TM helical domains (C), the overlay of 2D-NCA spectra of free and SspA C-pep–bound SGO_1180 (D), the refocused INEPT (rINEPT) of free and SspA C-pep–bound SGO_1180 to capture the dynamic residues of SGO_1180, and the 2D-rINEPT spectrum of SGO_1180 alone to detect the dynamic residues. Red, SGO_1180 alone. Black, SGO_1180 + SspA C-pep (1:1).
