Abstract
Background
The incidence of type 1 diabetes mellitus (T1DM) in children is increasing, resulting in higher burden of cardiovascular diseases due to diabetes mellitus–related vascular dysfunction.
Methods and Results
We examined cardiovascular risk factors (CVRFs) and arterial parameters in 1809 youth with T1DM. Demographics, anthropometrics, blood pressure, and laboratory data were collected at T1DM onset and 5 years later. Pulse wave velocity and augmentation index were collected with tonometry. ANOVA or chi‐square tests were used to test for differences in measures of arterial parameters by CVRF. Area under the curve of CVRFs was entered in general linear models to explore determinants of accelerate vascular aging. Participants at the time of arterial measurement were 17.6±4.5 years old, 50% female, 76% non‐Hispanic white, and duration of T1DM was 7.8±1.9 years. Glycemic control was poor (glycated hemoglobin, 9.1±1.8%). All arterial parameters were higher in participants with glycated hemoglobin ≥9% and pulse wave velocity was higher with lower insulin sensitivity or longer duration of diabetes mellitus. Differences in arterial parameters were found by sex, age, and presence of obesity, hypertension, or dyslipidemia. In multivariable models, higher glycated hemoglobin, lower insulin sensitivity, body mass index, blood pressure, and lipid areas under the curve were associated with accelerated vascular aging.
Conclusions
In young people with T1DM, persistent poor glycemic control and higher levels of traditional CVRFs are independently associated with arterial aging. Improving glycemic control and interventions to lower CVRFs may prevent future cardiovascular events in young individuals with T1DM.
Keywords: arterial compliance, diabetes mellitus, pediatric, risk factor
Subject Categories: Diabetes, Type 1; Cardiovascular Disease; Epidemiology; Pediatrics; Risk Factors
Clinical Perspective
What Is New?
The relationship between longitudinal burden of cardiovascular risk factors and objective measures of vascular health in youth with type 1 diabetes mellitus (T1DM) has not been previously explored.
Poor glycemic control over time is a major determinant of accelerated vascular aging in adolescents with T1DM.
Traditional cardiovascular risk factors including obesity, hypertension, and dyslipidemia also contribute to vascular aging in youth with T1DM.
What Are the Clinical Implications?
Tighter glycemic control needs to be achieved to reduce the burden of micro‐ and macrovascular disease in young people with T1DM.
Individuals with T1DM should strive to increase the number of ideal cardiovascular health metrics they are achieving as adverse levels of traditional cardiovascular risk factors also contribute to vascular aging in youth with T1DM.
Diabetes mellitus is one of the most common chronic diseases in children1, 2 affecting nearly 1 of every 433 youth in the United States,3and the majority of diabetes mellitus cases in childhood are due to type 1 diabetes mellitus (T1DM).4 As the rate of T1DM continues to climb,3, 5, 6 this may eventually result in higher cardiovascular diseases (CVD) incidence, and CVD is one of the major causes of death in adults with diabetes mellitus.7 One mechanism for the increase in CVD may be diabetes mellitus–related abnormalities in vascular structure and function that have been found in adolescents with diabetes mellitus.8, 9, 10 However, the true prevalence of these abnormalities in youth with T1DM is not known. It is also necessary to elucidate the correlates of vascular dysfunction in young patients with T1DM, which may guide future diabetes mellitus management recommendations. The presence of cardiac autonomic neuropathy (CAN) has also been associated with higher arterial stiffness in both adults and youth with diabetes mellitus, and it could be a potential contributor to vascular aging. Furthermore, analyses have examined the relationship between cardiovascular risk factors (CVRFs) measured at one point in time and arterial health in youth,11 but CVRF levels vary over time, and few studies have examined the long‐term burden of CVRFs on the vasculature in youth with T1DM. Therefore, our aim was to demonstrate that higher burden (area under the curve over time) of CVRFs was associated with measures of arterial stiffness and wave reflections in a cohort of individuals with onset of T1DM before 20 years of age. We also sought to determine if CAN was an independent predictor of arterial parameters.
Methods
In accordance with the Transparency and Openness Promotion Guidelines, the data that support the findings of this study are available from the statistical coordinating center (Department of Biostatistical Sciences, Wake Forest School of Medicine, Winston‐Salem, NC; sisom@wakehealth.edu; rdagosti@wakehealth.edu) upon reasonable request.
Description of the Study Population
Participants who were diagnosed with T1DM prior to 20 years of age who participated in a population‐based diabetes mellitus registry at 5 US sites by the SEARCH for Diabetes in Youth Study were eligible.2 Specifically, we recruited youth with newly diagnosed T1DM in 2002 through 2006 or 2008, who completed a SEARCH baseline examination for CVRFs ≈9 months after diagnosis and were reexamined at a follow up visit at least 5 years after the baseline examination. The average time from diagnosis to collection of arterial stiffness measures was 7.9±1.9 years. Nearly 60% of participants had one or more additional CVRF assessments between the baseline and follow‐up where vascular measures were obtained. Diabetes mellitus type was defined at the baseline visit using a classification developed by SEARCH12, 13 on the basis of ≥1 positive diabetes mellitus autoantibodies (see below) and estimated insulin sensitivity score (IS, validated equation including waist circumference, glycated hemoglobin [HbA1c] and triglyceride levels).12 T1DM was defined as the presence of at least 1 positive antibody, regardless of insulin sensitivity, or no positive antibodies and insulin sensitivity (score ≥8.15). This study included participants with T1DM, as defined above, with at least 1 measure of arterial stiffness, and C‐reactive protein levels ≤10 mg/Dl, as acute infection may transiently affect arterial stiffness. The final analytic sample consisted of 1809 participants. Institutional review board approval was obtained at each site, and all participants or their guardians gave informed consent.
CVRF Collection
All testing was performed under standardized conditions by trained personnel. At baseline and at the follow‐up visit, participants were examined after an 8‐hour overnight fast while refraining from strenuous exercise, smoking, or any caffeinated drinks. Short‐acting insulin and oral medications were withheld the morning of the visit until after the blood draw and vascular studies were complete. Race/ethnicity was self‐reported using a 2000 US Census–based questionnaire and was classified as non‐Hispanic white, non‐Hispanic black, Hispanic, and Other. Height was measured in centimeters using a stadiometer and weight in kilograms using a standardized scale. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters, and age and sex‐specific BMI z scores were derived on the basis of the 2000 Centers for Disease Control and Prevention national standards.14 Waist circumference was measured using the National Health and Nutrition Examination Survey protocol and divided by height in centimeters to calculate waist‐to‐height ratio.3 Resting systolic blood pressure (SBP) and diastolic blood pressure were measured 3 times, using an aneroid sphygmomanometer and an appropriate‐sized cuff, after the participants were seated for at least 5 minutes according to published guidelines.15 The average of multiple measures of anthropometric and blood pressure (BP) variables were used at each visit. Plasma samples were analyzed for HbA1c, low‐density lipoprotein cholesterol, high‐density lipoprotein (HDL) cholesterol, and triglyceride levels at the study central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington) as previously described.16 The study central laboratory measured the diabetes mellitus autoantibodies glutamic acid decarboxylase and insuloma‐associated‐2 antibody,17 while zinc‐T8 autoantibody was analyzed at the Eisenbarth Laboratory (University of Colorado, Aurora, CO).18
Arterial Parameters
At the follow‐up visit, the average of 3 measurements of each modality was obtained in a room with a stable temperature after the participant rested for >10 minutes using previously published protocols.10, 19 Pulse wave velocity (PWV) was collected using the SphygmoCor CPVH (AtCor Medical, Sydney, Australia) from the carotid to the femoral artery (PWVcf) representing central arterial stiffness in a large elastic artery. Carotid to radial (PWVcr) and femoral to foot (PWVft) were also obtained as measures of peripheral (muscular) artery stiffness. Briefly, ECG‐gated pressure wave data were obtained with a tonometer placed on the artery. PWV is the difference in proximal‐to‐distal artery path length (suprasternal notch to femoral artery measured directly with a caliper minus suprasternal notch to carotid pulse measured with a tape measure) divided by the difference in timing from the peak of the ECG R‐wave to foot of the proximal or distal pressure wave. Augmentation index (AIx), a measure of wave reflections influenced by vascular tone, heart rate, and cardiac function, was collected with the same device. For AIx, the pressure waves were calibrated by using mean arterial pressure (MAP) and diastolic BP obtained in the same arm. Using MAP instead of SBP is less prone to errors in calibration attributable to pulse pressure amplification along the brachial artery.20 A validated generalized transfer function was used to calculate central aortic pressure and AIx (augmentation of central pressure indexed to pulse pressure and adjusted to a heart rate of 75 bpm). These techniques are highly reproducible with coefficients of variability of 7% for PWV and intraclass correlation coefficients of 0.7 to 0.9 for AIx.10 For both PWV and AIx, a higher value indicates a stiffer vessel. To define increased arterial stiffness, the 90th percentile for all measures in healthy, lean adolescents and young adults of similar age range, obtained via the same technique in our laboratory, was used. The prevalence of increased stiffness (≥90th percentile) was determined.
Assessment of CAN
At follow‐up, presence of CAN was assessed by heart rate variability testing using the SphygmoCor device (Atcor Medical Inc, Sydney, Australia) as previously described.9 A 10‐minute continuous ECG recording was obtained in the supine position after a 5‐minute rest. ECG tracings were examined to ensure that R‐waves were adequately identified from artifacts and ectopic beats. The device analyzed time‐ and frequency‐domain parameters including SD of NN interval, root mean square difference of the successive NN interval, high‐frequency power, low‐frequency power, and low frequency–to–high frequency ratio. The heart rate variability test was considered abnormal if the values were below the fifth percentile observed in age‐ and sex‐matched healthy controls (age 10–28 years, 54% females) from the SEARCH CVD study.21 CAN was defined as presence of >3 abnormal heart rate variability indices.
Statistical Analysis
In participants aged ≥18 years, normal or underweight was defined as BMI<25 kg/m2, overweight as BMI 25 to 29.99 kg/m2, and obesity as BMI ≥30 kg/m2. In participants aged <18 years, normal or underweight was defined as age‐ and sex‐specific BMI <85th percentile. Overweight was defined as BMI at the 85th to 94th percentile, and obesity was defined as a BMI ≥95th percentile.22 Hypertension was defined as being on BP‐lowering medication; BP ≥140/90 mm Hg for young adults23 or ≥95th percentile for age, sex, and height for adolescents.15 Dyslipidemia was defined as being on lipid‐lowering medication or low‐density lipoprotein ≥130 mg/dL, HDL ≤40 mg/dL, triglycerides ≥150 mg/dL, or non‐HDL cholesterol ≥145 mg/dL.24 Smoking status was obtained by self‐report and categorized as never, current, or former smoker as previously described.25 Physical activity status was defined as participating in moderate or vigorous activity for at least 20 minutes at least 3 or more times per week. Sedentary behavior was defined as having >2 hours/day of screen time regardless of physical activity pattern.
The longitudinal cumulative exposure to CVRFs for each participant was calculated as the weighted average of the measures of interest during the participant's time in the study for waist‐to‐height ratio, SBP, and diastolic BP z scores, MAP, and non‐HDL cholesterol (potentially collected at baseline and 1‐, 2‐, and 5‐year follow‐up visits and weighted for the interval between each measurement, similar to the area under the curve [AUC]).
Statistical analyses were performed using SAS for Windows (version 9.4; SAS Institute, Cary, NC), and P<0.05 was considered significant. Mean (SD) or median (minimum, maximum) as appropriate was used for continuous variables and counts (percentages) for categorical variables were calculated for the entire study population. Participants were categorized by demographics, duration of T1DM, and various CVRFs at follow‐up (presence of obesity, hypertension, dyslipidemia, and lifestyle behaviors including smoking, physical activity and sedentary behavior levels). ANOVA or chi‐square tests were used to test for differences in measures of arterial stiffness and wave reflections in individuals with or without an adverse CVRF. Bivariate correlations between CVRFs, AUC for CVRFs, and arterial stiffness and wave reflection measures were obtained to guide development of multivariable general linear models (as all variables were continuous) to explore factors associated with increased arterial stiffness and wave reflections in the population. Variables with a skewed distribution were log‐transformed. The first models constructed adjusted for age at diabetes mellitus diagnosis, sex, race/ethnicity, clinic site, duration of diabetes mellitus, and 1 CVRF at a time. CVRF interactions with HbA1c AUC were also evaluated in the first series of models. The final models included all covariates, and reduced models retained only significant covariates.
Results
At the follow‐up visit, the cohort (Table 1, N=1809) was 17.6±4.5 years old (range, 6–30) and 50% were female, 76% non‐Hispanic white, 10% non‐Hispanic black, 12% Hispanic, and 2% Other. The mean duration of T1DM was 7.8±1.9 years (range, 5–13). The mean BMI was 23.9±5.2 kg/m2, although 26% of the participants were overweight and 14% had obesity. Mean BP was 106/68±11/9 mm Hg within the normal range, with 13% classified as having hypertension, including 6% on BP‐lowering medication. Mean lipid values were also within the normal range, although 29% were categorized as having dyslipidemia and 3% were on lipid‐lowering medication. Glycemic control was poor, with mean HbA1c 9.1±1.8% (range, 4–16). The mean daily dose of insulin was 0.9 (±0.4 units/kg body weight/day). While 68% of participants had never smoked and 58% reported adequate levels of physical activity, 92% reported high levels of sedentary behavior (data not shown).
Table 1.
Description of the Study Population at Follow‐Up (N=1809)
| Parameter | Mean (SD), Median (min, max), or Percent |
|---|---|
| Age, y | 17.6 (4.5) |
| Sex (% female) | 49.5 |
| Race/Ethnicity (%) | |
| Non‐Hispanic white | 76.2 |
| Non‐Hispanic black | 9.7 |
| Hispanic | 11.8 |
| Other | 2.4 |
| Duration of T1DM, y | 7.8 (1.9) |
| Height, m | 1.7 (0.1) |
| Weight, kg | 66.5 (19.6) |
| Waist‐to‐height ratio | 0.5 (0.1) |
| Body mass index, kg/m2 | 23.9 (5.2) |
| Body mass index (z score) AUC | 0.6 (0.9) |
| Systolic blood pressure, mm Hg | 106.0 (11.0) |
| Systolic blood pressure (z score) AUC | −0.4 (0.9) |
| Diastolic blood pressure, mm Hg | 68.4 (8.9) |
| Diastolic blood pressure (z score) AUC | 0.0 (1.1) |
| MAP (z score) AUC | 78.3 (7.4) |
| Heart rate, bpm | 69.5 (11.7) |
| Total cholesterol, mg/dL | 169.7 (34.8) |
| LDL‐cholesterol, mg/dL | 96.2 (28.1) |
| Triglycerides, mg/dL | 73.0 (17.0, 992.0) |
| HDL‐cholesterol, mg/dL | 55.2 (13.6) |
| HDL‐cholesterol (mg/dL) AUC | 56.0 (11.7) |
| Non–HDL‐cholesterol, mg/dL | 114.4 (34.7) |
| Non–HDL‐cholesterol (mg/dL) AUC | 109.4 (26.3) |
| Fasting glucose, mg/dL | 212.8 (92.2) |
| Insulin dose (units/kg of body weight per day) | 0.9 (0.4) |
| Glycated hemoglobin, % | 9.1 (1.8) |
| Glycated hemoglobin (%) AUC | 8.5 (1.3) |
| C‐reactive protein, mg/dL | 0.1 (0.0, 7.9) |
AUC indicates area under the curve; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MAP, mean arterial pressure; T1DM, type 1 diabetes mellitus.
Mean levels of arterial stiffness and wave reflections with participants stratified by a single CVRF at follow‐up are displayed in Table 2. The prevalence of increased wave reflections or arterial stiffness (value ≥90th percentile for lean, healthy youth) was 7.0% for AIx, 19.4% for PWVcf, 13.0% for PWVft, and 12.4% for PWVcr. All measures of arterial stiffness and wave reflections were higher in individuals with HbA1c ≥9% compared with those with better glycemic control (P≤0.05, Figure). These remained significant after adjusting for MAP, age, and sex for all measures except PWVcf (Figure S1). All measures of PWV were higher in participants with lower insulin sensitivity or longer duration of diabetes mellitus. PWVcf and PWVft were significantly higher in people classified as having CAN with a nonsignificant trend for PWVcr. In analyses stratified by levels of other CVRFs, differences in arterial parameters were found by sex (higher AIx in females, higher PWVft and PWVcr in males) and race (highest in non‐Hispanic blacks for all measures). Higher arterial parameters were also found for older individuals at follow‐up for all measures except wave reflections (AIx), which are lower with greater height with age, and in those with obesity (PWVcf), hypertension (all PWV), or dyslipidemia (PWVcf and PWVft); higher C‐reactive protein (AIx, PWVcf, and PWVft), reported smoking (PWV); and lower physical activity levels (all measures) with a trend for higher sedentary behavior (P=0.052 for PWVcf). Patterns were similar among all measures of arterial stiffness and wave reflections (see Tables S2 through S5 where differences were seen with 7, 14, 14, and 10 CVRFs for AIx, PWVcf, PWVft, and PWVcr, respectively).
Table 2.
Arterial Stiffness Overall and Stratified by Demographic and Clinical Characteristics at Follow‐Up, Mean (SE)*
| Covariate | AIx (%) | PWVcf (m/s) | PWVft (m/s) | PWVcr (m/s) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SE) | P Value | Mean (SE) | P Value | Mean (SE) | P Value | Mean (SE) | P Value | |
| Overall | −2.33 (0.28) | 5.44 (0.03) | 8.09 (0.03) | 7.42 (0.03) | ||||
| Age | 0.007 | <0.0001 | <0.0001 | <0.0001 | ||||
| Older (≥18) | −3.08 (0.39) | 5.87 (0.04) | 8.54 (0.05) | 7.67 (0.05) | ||||
| Younger (<18) | −1.60 (0.39) | 5.06 (0.03) | 7.69 (0.04) | 7.19 (0.04) | ||||
| Age at diagnosis | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Older (10–19) | −3.49 (0.38) | 5.80 (0.04) | 8.42 (0.05) | 7.57 (0.05) | ||||
| Younger (<10) | −1.09 (0.40) | 5.08 (0.03) | 7.77 (0.05) | 7.27 (0.04) | ||||
| Sex | <0.0001 | NS | <0.0001 | 0.01 | ||||
| Female | 0.47 (0.39) | 5.44 (0.04) | 7.89 (0.05) | 7.34 (0.05) | ||||
| Male | −4.99 (0.37) | 5.44 (0.04) | 8.28 (0.05) | 7.50 (0.04) | ||||
| Race | <0.0001 | <0.0001 | 0.0003 | <0.0001 | ||||
| Non‐Hispanic white | −3.01 (0.31) | 5.37 (0.03) | 8.07 (0.04) | 7.33 (0.04) | ||||
| Non‐Hispanic black | 0.97 (1.01) | 5.72 (0.09) | 8.45 (0.13) | 8.12 (0.11) | ||||
| Hispanic | −0.33 (0.76) | 5.58 (0.08) | 7.86 (0.10) | 7.36 (0.09) | ||||
| Other | −2.73 (1.38) | 5.79 (0.23) | 8.41 (0.23) | 7.57 (0.16) | ||||
| Glycated hemoglobin | 0.0001 | <0.0001 | 0.005 | 0.006 | ||||
| <9 | −3.35 (0.39) | 5.33 (0.04) | 8.00 (0.05) | 7.33 (0.04) | ||||
| ≥9 | −1.22 (0.39) | 5.56 (0.04) | 8.19 (0.05) | 7.51 (0.05) | ||||
| Insulin sensitivity | NS | <0.0001 | <0.0001 | 0.01 | ||||
| <8.15 (insulin resistant) | −2.48 (0.32) | 5.63 (0.03) | 8.20 (0.04) | 7.47 (0.04) | ||||
| 8.15+ (insulin sensitive) | −2.07 (0.56) | 4.96 (0.04) | 7.81 (0.06) | 7.29 (0.06) | ||||
| Duration of diabetes mellitus | NS | <0.0001 | <0.0001 | 0.002 | ||||
| <8 y | −2.63 (0.39) | 5.26 (0.03) | 7.90 (0.05) | 7.33 (0.05) | ||||
| ≥8 y | −2.02 (0.39) | 5.65 (0.04) | 8.31 (0.05) | 7.53 (0.04) | ||||
| Cardiac autonomic neuropathy | NS | 0.0002 | 0.0005 | |||||
| No | −2.75 (0.30) | 5.45 (0.03) | 8.08 (0.04) | 7.42 (0.04) | 0.09 | |||
| Yes | −2.68 (0.85) | 5.76 (0.08) | 8.46 (0.11) | 7.60 (0.09) | ||||
AIx indicates augmentation index; NS, nonsignificant; PWVcf, pulse wave velocity, carotid‐femoral; PWVcr, pulse wave velocity, carotid‐radial; PWVft, pulse wave velocity, femoral‐foot.
Figure 1.
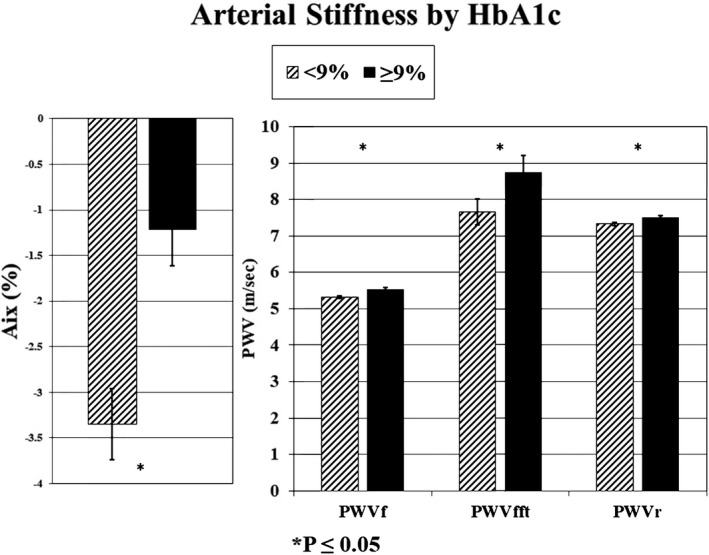
Arterial stiffness means with standard error bars by glycosylated hemoglobin (HbA1c levels, %) in youth and young adults with type 1 diabetes mellitus. AIx indicates augmentation index; PWVcf, pulse wave velocity, carotid‐femoral; PWVcr, pulse wave velocity, carotid‐radial; PWVft, pulse wave velocity, femoral‐foot.
Generalized linear models exploring the association of the burden of CVRFs over time on measures of arterial stiffness and wave reflections are presented in Table 3. For each of the CVRF AUCs, a model was adjusted for demographics, clinic site, and duration of diabetes mellitus. Higher HbA1c AUC was associated with adverse levels of all vascular parameters. A measure of adiposity (either higher BMI and/or waist‐to‐height ratio) or a measure of BP (higher SBP, diastolic BP, and/or MAP) or lipids (lower HDL and/or higher non‐HDL cholesterol) was associated with higher stiffness and wave reflections. Presence of CAN was significant for PWVcf only after adjustments. The models in Table 3 were repeated with the addition of adjustment for HbA1c AUC or log(IS) AUC (data not shown). Adjusting for HbA1c resulted in no changes, except non‐HDL cholesterol was no longer statistically significantly associated with PWVcr.
Table 3.
Association of Arterial Stiffness With AUC of Metabolic and Cardiovascular Risk Factors in Youth and Young Adults with T1DMa
| Cardiovascular Risk Factor Included in the Model | AIx | PWVcf | PWVft | PWVcr | ||||
|---|---|---|---|---|---|---|---|---|
| Beta (SE) | P Value | Beta (SE) | P Value | Beta (SE) | P Value | Beta (SE) | P Value | |
| HbA1c (%) AUC | 0.97 (0.20) | <0.0001 | 0.07 (0.02) | 0.0002 | 0.09 (0.03) | 0.0002 | 0.08 (0.02) | 0.0005 |
| BMI z score AUC | 0.05 (0.30) | NS | 0.23 (0.02) | <0.0001 | −0.10 (0.04) | 0.007 | −0.09 (0.03) | 0.01 |
| WHR AUC | 24.95 (4.45) | <0.0001 | 4.12 (0.39) | <0.0001 | −0.38 (0.57) | NS | −0.15 (0.50) | NS |
| SBP z score AUC | 1.44 (0.32) | <0.0001 | 0.13 (0.03) | <0.0001 | 0.10 (0.04) | 0.009 | 0.07 (0.04) | 0.08 |
| DBP z score AUC | 1.40 (0.26) | <0.0001 | 0.09 (0.02) | 0.0003 | 0.07 (0.03) | 0.02 | 0.11 (0.03) | 0.0005 |
| MAP (mm Hg) AUC | 0.05 (0.04) | NS | 0.03 (0.00) | <0.0001 | 0.03 (0.01) | <0.0001 | 0.02 (0.00) | 0.0006 |
| HDL (mg/dL) AUC (10 mg/dL change) | −0.03 (0.24) | NS | −0.05 (0.02) | 0.02 | −0.01 (0.03) | NS | 0.02 (0.03) | NS |
| Non‐HDL AUC (10 mg/dL change) | 0.47 (0.11) | <0.0001 | 0.05 (0.01) | <0.0001 | 0.01 (0.01) | NS | 0.03 (0.01) | 0.02 |
| CAN (yes vs no) | 1.12 (0.82) | NS | 0.13 (0.07) | 0.08 | 0.21 (0.10) | 0.050 | 0.12 (0.10) | NS |
AIx indicates augmentation index; AUC, area under the curve; BMI, body mass index; CAN, cardiac autonomic neuropathy; DBP, diastolic blood pressure; HbA1c, glycated hemoblobin; HDL, high‐density lipoprotein; MAP, mean arterial pressure; NS, nonsignificant; PWVcf, pulse wave velocity, carotid‐femoral; PWVcr, pulse wave velocity, carotid‐radial; PWVft, pulse wave velocity, femoral‐foot; SBP, systolic blood pressure; T1DM, type 1 diabetes mellitus; WHR, waist‐to‐height ratio.
Generalized linear models for each cardiovascular risk factor AUC adjusted for age at diagnosis, sex, race, clinic site, duration of diabetes mellitus, and 1 cardiovascular risk factor AUC.
Full models including all CVRFs, adjusted for clinic site and race, are presented in Table 4 (models with standardized beta estimates are found in Table S1). HbA1c AUC was statistically significantly associated with all arterial measures, except there was only a trend for PWVcr. There was a HbA1c AUC by non‐HDL interaction term that entered the model for AIx, HbA1c by CAN for AIx and PWVft, and HbA1c by waist‐to‐height ratio term for PWVcf. Duration of T1DM was positively associated with higher stiffness or wave reflections. A measure of adiposity (for all measures except PWVcr) and MAP entered all the PWV models. Non‐HDL cholesterol was significantly associated with AIx and PWVcf. CAN was not significant for any measure. Use of BP or lipid‐lowering medication, smoking, and sedentary behavior were not significant, and being physically active was significantly associated with PWVft and PWVcr (in a favorable direction). Removal from the model of variables that did not demonstrate a statistically significant association did not change the R 2 substantially but did result in a significant association of HbA1c and non‐HDL with PWVcr (data not shown).
Table 4.
Multivariable Associations of Metabolic and CVRFs with Arterial Stiffness in Youth and Young Adults With T1DMa
| Parameter | AIx | PWVcf | PWVft | PWVcr | ||||
|---|---|---|---|---|---|---|---|---|
| Beta (SE) | P Value | Beta (SE) | P Value | Beta (SE) | P Value | Beta (SE) | P Value | |
| HbA1c (%) AUC | 4.08 (1.04) | 0.0004 | −0.32 (0.15) | 0.03 | −0.01 (0.07) | 0.94 | 0.05 (0.03) | 0.08 |
| Duration of T1DM, y | 0.62 (0.16) | <0.0001 | 0.10 (0.01) | <0.0001 | 0.11 (0.02) | <0.0001 | 0.06 (0.02) | 0.003 |
| Waist circumference AUCa | 0.06 (0.03) | 0.03 | ||||||
| Waist‐to‐height ratio AUC | −1.58 (0.66) | 0.02 | ||||||
| MAP (mm Hg) AUC | 0.02 (0.00) | <0.0001 | 0.03 (0.01) | <0.0001 | 0.02 (0.01) | 0.0002 | ||
| Non‐HDL AUC (10 mg/dL increase) | 1.87 (0.67) | 0.01 | 0.02 (0.01) | 0.05 | 0.01 (0.01) | NS | 0.02 (0.01) | 0.07 |
| CAN: N (Y is ref) | 13.36 (5.28) | 0.01 | −0.13 (0.08) | 0.09 | −1.13 (0.70) | 0.11 | −0.08 (0.10) | NS |
| Physically active: N (Y is ref) | 0.48 (0.55) | NS | 0.05 (0.05) | NS | 0.15 (0.07) | 0.04 | 0.15 (0.07) | 0.03 |
| HbA1c (%) AUC×Non‐HDL AUC (10 mg/dL increase) | −0.19 (0.08) | 0.01 | ||||||
| HbA1c (%) AUC×CAN: N (Y is ref) | −1.64 (0.60) | 0.006 | 0.11 (0.08) | 0.15 | ||||
| HbA1c (%) AUC×waist‐to‐height ratio AUC | 0.75 (0.30) | 0.01 | ||||||
| Model R 2 | 0.23 | 0.30 | 0.18 | 0.14 | ||||
| Reduced model R 2 | 0.22 | 0.29 | 0.18 | 0.14 | ||||
This reduced model excludes the variables that were not significant. AIx indicates augmentation index; AUC, area under the curve; CAN, cardiac autonomic neuropathy; HbA1c, glycated hemoblobin; HDL, high‐density lipoprotein; MAP, mean arterial pressure; PWVcf, pulse wave velocity, carotid‐femoral; PWVcr, pulse wave velocity, carotid‐radial; PWVft, pulse wave velocity, femoral‐foot; T1DM, type 1 diabetes mellitus.
In generalized linear models, all variables listed, age, sex, race, use of blood pressure or lipid medication, smoking, sedentary behavior, and clinic site were included simultaneously. For AIx, model was adjusted for height at the time of the measure and waist AUC, other outcomes use the National Health and Nutrition Examination Survey waist‐to‐height ratio AUC.
Discussion
Our data demonstrate that in adolescents and young adults, the duration of T1DM and glycemic control over time (HbA1c AUC) are consistent determinants of association with arterial stiffness and wave reflections regardless of sex and race and after adjustment for other known CVRFs. However, increased adiposity, higher BP, and adverse lipid levels were also important correlates, suggesting that T1DM does affect the vasculature but traditional CVRFs are also important. In our previous work in a substudy of SEARCH (N=298), we also found that CVRFs were important in predicting change in PWVcf.11 These new data extend the observations to a larger cohort with more racial and ethnic diversity that includes additional arterial stiffness and wave reflection parameters and examines the burden of risk factors over time (AUC).
Studies in adults have examined the relationship between presence and control of T1DM and arterial stiffness and wave reflections. One study of 89 adults (mean age, 34 years) found that diabetes mellitus was an important determinant of Aix, but the relationship was stronger in men and an interaction with age suggested that duration of disease also had an influence.26 In a smaller case‐control study, AIx was significantly higher in participants with T1DM as compared with controls (7.1±1.6% versus 0.4±−2.0%; P=0.01).27 They also estimated PWV by calculating the time from the foot of the aortic pressure wave to the inflection point and found this higher in adults with diabetes mellitus.27 However, when PWV was directly measured in another study, no significant difference in PWV was seen between T1DM cases and controls, although this study suffered from a small sample size (N=17 with T1DM).28 Other investigators have found higher PWV in participants with T1DM,29, 30 consistent with our previous work in the SEARCH study where we demonstrated higher AIx and PWV in adolescents and young adults (mean age, 17.8 years) with T1DM as compared with controls,10 and presence of diabetes mellitus remained a significant correlate even after adjusting for other CVRFs.31 In the SEARCH CVD substudy, higher HbA1c was significantly associated with change in only carotid‐femoral PWV over time.11 Our current study is able to demonstrate the effect of burden of glycemic dyscontrol over time on a variety of vascular parameters.
The relationship between duration of diabetes mellitus and arterial stiffness and wave reflections has also been examined. Adults with T1DM for >10 years had higher PWV than those with shorter exposure to disease, with duration and SBP remaining important determinants in multivariable analyses.32 In a small study of 30 children with T1DM, PWV correlated with duration of diabetes mellitus, but AIx did not.33 Our previous study (N=535) found a relationship between duration and both AIx and PWV.34
The effect of metabolic control and arterial stiffness and wave reflections is complex. Although induction of acute hyperglycemia was found to increase both PWV and AIx in adults with T1DM,35 no relationship was found between chronic glycemic control (HbA1c) and AIx in a study that included only adult women.36 When we looked at glycemic control in younger adolescents (mean age, 14.6 years) of both sexes with T1DM, we also found that although HbA1c correlates with arterial stiffness and wave reflections, it was not a significant determinant of AIx or PWV once adjusted for BP and lipids.34 Similarly, in children with T1DM, no relationship was seen between HbA1c and ultrasound‐based abdominal aortic distensibility, a measure that correlates with PWV.37 However, in a study using magnetic resonance imaging, perhaps a more precise measure than ultrasound, an inverse relationship was found between HbA1c and aortic distensibility in adolescents with T1DM.38 Furthermore in a previous smaller substudy of the SEARCH study, we examined 298 adolescents with T1DM from 2 of the SEARCH sites who had CVRFs and PWV at baseline (mean age, 14.5 years) and after 5 years of follow up. A lower insulin sensitivity score, which contains HbA1c in the calculation, was associated with a greater rate of change in PWV over 5 years.11 These observations along with our current work suggest that there is a relationship between glycemic control and arterial stiffness, but the effect is modest, requiring a larger sample size or longer duration of follow‐up to detect.
In adults, adiposity and BP have been found to be important predictors of arterial parameters.31, 39 Pediatric studies also show a direct relationship between greater AIx and higher BMI,40 and higher PWV and higher lipid and BP levels.41 Previous SEARCH analyses confirm these relationships, demonstrating that adiposity, BP, and lipids are determinants of higher arterial stiffness in cross‐sectional analyses31, 42 and contribute to increasing arterial stiffness over time.11 Our current work extends these observations by demonstrating the effect of burden of risk factors over time (using AUC) on arterial stiffness in young adults with T1DM.
There are numerous mechanisms by which poor metabolic control may affect vascular parameters. Animal models have shown that in the setting of insulin resistance, there is reduced activation of the nitric oxide signaling pathway (vasodilating) and enhanced activation of the endothelin pathway (vasoconstricting), resulting in increased vascular tone.43 Because nitric oxide attenuates production of proinflammatory cytokines, insulin resistance also leads to an inflammatory milieu and increased reactive oxygen species, which also contribute to vascular dysfunction.44 Hyperglycemia also leads to formation of advanced glycation end products, which may result in abnormal cross‐linking of collagen and elastin fibers.45 These advanced glycation end products were independently associated with PWV in adults with T1DM.46 Diabetes mellitus is also associated with development of CAN47 with concomitant sympathetic predominance, resulting in increased arterial stiffness in adults with T1DM.48, 49, 50 SEARCH investigators have demonstrated a similar relationship in youth with T1DM between reduced heart rate variability, reflecting CAN, and increased arterial stiffness.9 Our data suggest that the burden of CAN over time has only a modest effect on arterial stiffness when other CVRFs are taken into account.
Limitations
Our study examines the effect of CVRF burden on arterial parameters in young individuals with T1DM. Therefore, our findings may not be generalizable to healthy youth or those with other high‐risk conditions such as uncomplicated obesity, type 2 diabetes mellitus, hypertension, dyslipidemia, or chronic kidney disease. We were also unable to compare the effect of CVRFs on arterial parameters in people without diabetes mellitus because of constraints of the overall design of the parent SEARCH study, but case‐control comparisons were thoroughly explored in our ancillary SEARCH CVD study.31 Although we have CVRF data over time, we collected only arterial parameters measures at follow‐up, so we can only demonstrate association and cannot conclude that the risk factors caused any vascular dysfunction.
There are many controversies in the measurement of arterial parameters. The usefulness of AIx, a measure of wave reflection, to evaluate arterial health has been challenged51 because it may be affected by factors such as anatomy or peripheral vascular resistance, not directly related to arterial stiffness. Furthermore, the contribution of the forward and backward waves may be different,52 and we were not able to assess these features in the current study. However, a large meta‐analysis in adults demonstrated that higher AIx was associated with greater incidence of cardiovascular events53; therefore, we believe our examination of AIx in a young diabetic population adds to the literature. The relevance of the peripheral stiffness measures is not entirely clear, as no studies have evaluated the relationship between PWVcr or PWVft and cardiovascular events. These measures may be more sensitive to vascular tone. However, we include these measures because few studies have evaluated the relationship between arterial stiffness and peripheral artery disease,54 but limited studies in adults with diabetes mellitus suggest a relationship between PWV in the leg and presence of peripheral artery disease,55 and one group has found higher PWVcr in women with insulin resistance56 and offspring of adults with diabetes mellitus,57 so there may be utility in measuring PWV in the arm in diabetes mellitus. We also used the “subtraction” rather than “80% direct” method to measure arterial path length purported to be more accurate compared with magnetic resonance imaging.58 However, another larger study suggested the subtraction method is more accurate compared with magnetic resonance imaging.59
Conclusion
The major factors associated with wave reflections (AIx) and arterial stiffness (PWV) in young people with T1DM are duration of diabetes mellitus, glycemic control over time, and traditional CVRFs including obesity, BP, and lipids. Because presence of greater numbers of ideal cardiovascular health metrics in youth with T1DM is associated with lower arterial stiffness,42 efforts to improve glycemic control combined with interventions to lower CVRFs may prevent future cardiovascular events in young individuals with T1DM.
Sources of Funding
SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP‐05‐069, and DP‐10‐001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Site Contract Numbers: Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241‐3, U01 DP000247, and U18DP000247‐06A1), Children's Hospital Medical Center (Cincinnati) (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708), University of Washington School of Medicine (U58/CCU019235‐4, U01 DP000244, and U18DP002710‐01), Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200‐2010‐35171). The authors wish to acknowledge the involvement of the South Carolina Clinical & Translational Research Institute, at the Medical University of South Carolina, NIH/National Center for Advancing Translational Sciences (NCATS) grant number UL1 TR000062; Seattle Children's Hospital and the University of Washington, NIH/NCATS grant number UL1 TR00423; University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant Number UL1 TR000154; the Barbara Davis Center at the University of Colorado at Denver (DERC NIH grant number P30 DK57516); the University of Cincinnati, NIH/NCATS grant number UL1 TR000077; and the Children with Medical Handicaps program managed by the Ohio Department of Health. These studies were supported in part by Center for Disease Control and National Institute Of Diabetes And Digestive And Kidney Diseases (Grant/Award Number: UC4DK108173) to D’Agostino, Dolan, and Dabelea; and by Centers for Disease Control and Prevention (Grant/Award Number: U18DP006139) to Dabelea.
Disclosures
This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
Supporting information
Appendix S1.
Table S1. Multivariable Associations of Metabolic and CVRFs With Arterial Stiffness in Youth and Young Adults With T1DM (standardized beta estimates)*
Table S2. Generalized Linear Multivariable Models of Associations of Metabolic and CVRFs With Augmentation Index in Youth and Young Adults with T1DM*
Table S3. Generalized Linear Multivariable Models of Associations of Metabolic and CVRFs With Carotid‐Femoral Pulse Wave Velocity in Youth and Young Adults With T1DM*
Table S4. Generalized Linear Multivariable Models of Associations of Metabolic and CVRFs With Femoral‐Foot Pulse Wave Velocity in Youth and Young Adults with T1DM*
Table S5. Generalized Linear Multivariable Models of Associations of Metabolic and CVRFs With Carotid‐Radial Pulse Wave Velocity in Youth and Young Adults with T1DM*
Figure S1. Arterial Stiffness means with standard error bars by glycosylated hemoglobin (HbA1c levels, %) in youth and young adults with type 1 diabetes mellitus adjusted (for mean arterial pressure, age, and sex).
Acknowledgments
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their healthcare providers, whose participation made this study possible.
(J Am Heart Assoc. 2019;8:e010150 DOI: 10.1161/JAHA.118.010150.)
References
- 1. Liese AD, D'Agostino RB Jr, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, Loots B, Linder B, Marcovina S, Rodriguez B, Standiford D, Williams DE. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–1518. [DOI] [PubMed] [Google Scholar]
- 2. Hamman RF, Bell RA, Dabelea D, D'Agostino RB Jr, Dolan L, Imperatore G, Lawrence JM, Linder B, Marcovina SM, Mayer‐Davis EJ, Pihoker C, Rodriguez BL, Saydah S; the SEARCH for Diabetes in Youth Study Group . The SEARCH for Diabetes in Youth Study: rationale, findings, and future directions. Diabetes Care. 2014;37:3336–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pettitt DJ, Talton JW, Liese AD, Liu LL, Crimmins N, West NA, D'Agostino RB Jr, Kahn HS; the SEARCH for Diabetes in Youth Study Group . Comparison of two waist circumference measurement protocols: the SEARCH for Diabetes in Youth Study. Pediatr Obes. 2012;7:e81–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pettitt DJ, Talton J, Dabelea D, Divers J, Imperatore G, Lawrence JM, Liese AD, Linder B, Mayer‐Davis EJ, Pihoker C, Saydah SH, Standiford DA, Hamman RF; Group SfDiYS . Prevalence of diabetes in U.S. youth in 2009: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2014;37:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dabelea D, Bell RA, D'Agostino RB Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer‐Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. [DOI] [PubMed] [Google Scholar]
- 6. Mayer‐Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L; the SEARCH for Diabetes in Youth Study Group . Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benjamin E, Virani S, Callaway C, Chang A, Cheng S, Chiuve S, Cushman M, Delling F, Deo R, de Ferranti S, Ferguson J, Fornage M, Gillespie C, Isasi C, Jiménez M, Jordan L, Judd S, Lackland D, Lichtman J, Lisabeth L, Liu S, Longenecker C, Lutsey P, Matchar D, Matsushita K, Mussolino M, Nasir K, O'Flaherty M, Palaniappan L, Pandey D, Reeves M, Ritchey M, Rodriguez C, Roth G, Rosamond W, Sampson UA, Satou G, Shah S, Spartano N, Tirschwell D, Tsao C, Voeks J, Willey J, Wilkins J, Wu J, Alger H, Wong S, Muntner P. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e146–e603. [DOI] [PubMed] [Google Scholar]
- 8. Gordon SM, Davidson WS, Urbina EM, Dolan LM, Heink A, Zang H, Lu LJ, Shah AS. The effects of type 2 diabetes on lipoprotein composition and arterial stiffness in male youth. Diabetes. 2013;62:2958–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jaiswal M, Urbina EM, Wadwa RP, Talton JW, D'Agostino RB Jr, Hamman RF, Fingerlin TE, Daniels SR, Marcovina SM, Dolan LM, Dabelea D. Reduced heart rate variability is associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36:2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Urbina EM, Wadwa RP, Davis C, Snively BM, Dolan LM, Daniels SR, Hamman RF, Dabelea D. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr. 2010;156:731–737, 737.e1. [DOI] [PubMed] [Google Scholar]
- 11. Dabelea D, Talton JW, D'Agostino R Jr, Wadwa RP, Urbina EM, Dolan LM, Daniels SR, Marcovina SM, Hamman RF. Cardiovascular risk factors are associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36:3938–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dabelea D, D'Agostino RB Jr, Mason CC, West N, Hamman RF, Mayer‐Davis EJ, Maahs D, Klingensmith G, Knowler WC, Nadeau K. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth Study. Diabetologia. 2011;54:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dabelea D, Pihoker C, Talton JW, D'Agostino RB Jr, Fujimoto W, Klingensmith GJ, Lawrence JM, Linder B, Marcovina SM, Mayer‐Davis EJ, Imperatore G, Dolan LM. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34:1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control . Selected Z‐score values. 2017. Available at: https://www.cdc.gov/growthcharts/data_tables.htm. Accessed August 24, 2015.
- 15. Expert Panel . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. SEARCH Study Group . SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–471. [DOI] [PubMed] [Google Scholar]
- 17. Bonifacio E, Yu L, Williams AK, Eisenbarth GS, Bingley PJ, Marcovina SM, Adler K, Ziegler AG, Mueller PW, Schatz DA, Krischer JP, Steffes MW, Akolkar B. Harmonization of glutamic acid decarboxylase and islet antigen‐2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95:3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lampasona V, Schlosser M, Mueller PW, Williams AJ, Wenzlau JM, Hutton JC, Achenbach P. Diabetes antibody standardization program: first proficiency evaluation of assays for autoantibodies to zinc transporter 8. Clin Chem. 2011;57:1693–1702. [DOI] [PubMed] [Google Scholar]
- 19. Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity‐related type 2 diabetes mellitus. J Hypertens. 2010;28:1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rezai MR, Goudot G, Winters C, Finn JD, Wu FC, Cruickshank JK. Calibration mode influences central blood pressure differences between SphygmoCor and two newer devices, the Arteriograph and Omron HEM‐9000. Hypertens Res. 2011;34:1046–1051. [DOI] [PubMed] [Google Scholar]
- 21. Jaiswal M, Urbina EM, Wadwa RP, Talton JW, D'Agostino RB Jr, Hamman RF, Fingerlin TE, Daniels S, Marcovina SM, Dolan LM, Dabelea D. Reduced heart rate variability among youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, Urbina EM, Ewing LJ, Daniels SR. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;123:1689–1712. [DOI] [PubMed] [Google Scholar]
- 23. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 24. Maahs DM, Daniels SR, deFerranti SD , Dichek HL, Flynn J, Goldstein BI, Kelly AS, Nadeau KJ, Martyn‐Nemeth P, Osganian SK, Quinn L, Shah AS, Urbina E; American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing; Council for High Blood Pressure Research; Council on Lifestyle and Cardiometabolic Health . Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014;130:1532–1558. [DOI] [PubMed] [Google Scholar]
- 25. Shah AS, Dabelea D, Talton JW, Urbina EM, D Agostino RB Jr, Wadwa RP, Marcovina S, Hamman RF, Daniels SR, Dolan LM. Smoking and arterial stiffness in youth with type 1 diabetes: the SEARCH Cardiovascular Disease Study. J Pediatr. 2014;165:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brooks B, Molyneaux L, Yue DK. Augmentation of central arterial pressure in type 1 diabetes. Diabetes Care. 1999;22:1722–1727. [DOI] [PubMed] [Google Scholar]
- 27. Wilkinson IB, MacCallum H, Rooijmans DF, Murray GD, Cockcroft JR, McKnight JA, Webb DJ. Increased augmentation index and systolic stress in type 1 diabetes mellitus. QJM. 2000;93:441–448. [DOI] [PubMed] [Google Scholar]
- 28. Sweitzer NK, Shenoy M, Stein JH, Keles S, Palta M, LeCaire T, Mitchell GF. Increases in central aortic impedance precede alterations in arterial stiffness measures in type 1 diabetes. Diabetes Care. 2007;30:2886–2891. [DOI] [PubMed] [Google Scholar]
- 29. Stakos DA, Schuster DP, Sparks EA, Wooley CF, Osei K, Boudoulas H. Cardiovascular effects of type 1 diabetes mellitus in children. Angiology. 2005;56:311–317. [DOI] [PubMed] [Google Scholar]
- 30. Haller MJ, Pierce GL, Braith RW, Silverstein JH. Serum superoxide dismutase activity and nitric oxide do not correlate with arterial stiffness in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2006;19:267–269. [DOI] [PubMed] [Google Scholar]
- 31. Shah AS, Wadwa RP, Dabelea D, Hamman RF, D'Agostino R Jr, Marcovina S, Daniels SR, Dolan LM, Fino NF, Urbina EM. Arterial stiffness in adolescents and young adults with and without type 1 diabetes: the SEARCH CVD study. Pediatr Diabetes. 2015;16:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vastagh I, Horvath T, Nagy G, Varga T, Juhasz E, Juhasz V, Kollai M, Bereczki D, Somogyi A. Evolution and predictors of morphological and functional arterial changes in the course of type 1 diabetes mellitus. Diabetes Metab Res Rev. 2010;26:646–655. [DOI] [PubMed] [Google Scholar]
- 33. Heilman K, Zilmer M, Zilmer K, Lintrop M, Kampus P, Kals J, Tillmann V. Arterial stiffness, carotid artery intima‐media thickness and plasma myeloperoxidase level in children with type 1 diabetes. Diabetes Res Clin Pract. 2009;84:168–173. [DOI] [PubMed] [Google Scholar]
- 34. Wadwa RP, Urbina EM, Anderson AM, Hamman RF, Dolan LM, Rodriguez BL, Daniels SR, Dabelea D. Measures of arterial stiffness in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2010;33:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gordin D, Ronnback M, Forsblom C, Heikkila O, Saraheimo M, Groop PH. Acute hyperglycaemia rapidly increases arterial stiffness in young patients with type 1 diabetes. Diabetologia. 2007;50:1808–1814. [DOI] [PubMed] [Google Scholar]
- 36. Tryfonopoulos D, Anastasiou E, Protogerou A, Papaioannou T, Lily K, Dagre A, Souvatzoglou E, Papamichael C, Alevizaki M, Lekakis J. Arterial stiffness in type 1 diabetes mellitus is aggravated by autoimmune thyroid disease. J Endocrinol Invest. 2005;28:616–622. [DOI] [PubMed] [Google Scholar]
- 37. Galler A, Heitmann A, Siekmeyer W, Gelbrich G, Kapellen T, Kratzsch J, Kiess W. Increased arterial stiffness in children and adolescents with type 1 diabetes: no association between arterial stiffness and serum levels of adiponectin. Pediatr Diabetes. 2010;11:38–46. [DOI] [PubMed] [Google Scholar]
- 38. McCulloch MA, Mauras N, Canas JA, Hossain J, Sikes KM, Damaso LC, Redheuil A, Ross JL, Gidding SS. Magnetic resonance imaging measures of decreased aortic strain and distensibility are proportionate to insulin resistance in adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2015;16:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Llaurado G, Ceperuelo‐Mallafre V, Vilardell C, Simo R, Freixenet N, Vendrell J, Gonzalez‐Clemente JM. Arterial stiffness is increased in patients with type 1 diabetes without cardiovascular disease: a potential role of low‐grade inflammation. Diabetes Care. 2012;35:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zineh I, Beitelshees AL, Haller MJ. NOS3 polymorphisms are associated with arterial stiffness in children with type 1 diabetes. Diabetes Care. 2007;30:689–693. [DOI] [PubMed] [Google Scholar]
- 41. Bjornstad P, Nguyen N, Reinick C, Maahs DM, Bishop FK, Clements SA, Snell‐Bergeon JK, Lieberman R, Pyle L, Daniels SR, Paul Wadwa R. Association of apolipoprotein B, LDL‐C and vascular stiffness in adolescents with type 1 diabetes. Acta Diabetol. 2015;52:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alman AC, Talton JW, Wadwa RP, Urbina EM, Dolan LM, Daniels SR, Hamman RF, D'Agostino RB, Marcovina SM, Mayer‐Davis EJ, Dabelea DM. Cardiovascular health in adolescents with type 1 diabetes: the SEARCH CVD Study. Pediatr Diabetes. 2014;15:502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eringa EC, Stehouwer CD, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab. 2007;293:E1134–E1139. [DOI] [PubMed] [Google Scholar]
- 44. Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am. 2008;37:685–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aronson D. Cross‐linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. [DOI] [PubMed] [Google Scholar]
- 46. Llaurado G, Ceperuelo‐Mallafre V, Vilardell C, Simo R, Gil P, Cano A, Vendrell J, Gonzalez‐Clemente JM. Advanced glycation end products are associated with arterial stiffness in type 1 diabetes. J Endocrinol. 2014;221:405–413. [DOI] [PubMed] [Google Scholar]
- 47. Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Investig. 2013;4:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jensen‐Urstad K, Reichard P, Jensen‐Urstad M. Decreased heart rate variability in patients with type 1 diabetes mellitus is related to arterial wall stiffness. J Intern Med. 1999;245:57–61. [DOI] [PubMed] [Google Scholar]
- 49. Liatis S, Alexiadou K, Tsiakou A, Makrilakis K, Katsilambros N, Tentolouris N. Cardiac autonomic function correlates with arterial stiffness in the early stage of type 1 diabetes. Exp Diabetes Res. 2011;2011:957901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ. Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care. 2010;33:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fantin F, Mattocks A, Bulpitt CJ, Banya W, Rajkumar C. Is augmentation index a good measure of vascular stiffness in the elderly? Age Ageing. 2007;36:43–48. [DOI] [PubMed] [Google Scholar]
- 52. Hodson B, Norton GR, Ballim I, Sareli P, Woodiwiss AJ. Contribution of backward and forward wave pressures to age‐related increases in aortic pressure in a community sample not receiving antihypertensive therapy. J Am Soc Hypertens. 2017;11:616–626.e2. [DOI] [PubMed] [Google Scholar]
- 53. Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with central haemodynamics: a systematic review and meta‐analysis. European Heart J. 2010;31:1865–1871. [DOI] [PubMed] [Google Scholar]
- 54. Zagura M, Kals J, Kilk K, Serg M, Kampus P, Eha J, Soomets U, Zilmer M. Metabolomic signature of arterial stiffness in male patients with peripheral arterial disease. Hypertens Res. 2015;38:840–846. [DOI] [PubMed] [Google Scholar]
- 55. Yokoyama H, Shoji T, Kimoto E, Shinohara K, Tanaka S, Koyama H, Emoto M, Nishizawa Y, Yokoyama H, Shoji T, Kimoto E, Shinohara K, Tanaka S, Koyama H, Emoto M, Nishizawa Y. Pulse wave velocity in lower‐limb arteries among diabetic patients with peripheral arterial disease. J Atheroscler Thromb. 2003;10:253–258. [DOI] [PubMed] [Google Scholar]
- 56. Kelly CJ, Speirs A, Gould GW, Petrie JR, Lyall H, Connell JM. Altered vascular function in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:742–746. [DOI] [PubMed] [Google Scholar]
- 57. McEleavy OD, McCallum RW, Petrie JR, Small M, Connell JM, Sattar N, Cleland SJ. Higher carotid‐radial pulse wave velocity in healthy offspring of patients with type 2 diabetes. Diabet Med. 2004;21:262–266. [DOI] [PubMed] [Google Scholar]
- 58. Huybrechts SA, Devos DG, Vermeersch SJ, Mahieu D, Achten E, de Backer TL, Segers P, van Bortel LM. Carotid to femoral pulse wave velocity: a comparison of real travelled aortic path lengths determined by MRI and superficial measurements. J Hypertens. 2011;29:1577–1582. [DOI] [PubMed] [Google Scholar]
- 59. Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age‐associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. 2008;1:739–748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Table S1. Multivariable Associations of Metabolic and CVRFs With Arterial Stiffness in Youth and Young Adults With T1DM (standardized beta estimates)*
Table S2. Generalized Linear Multivariable Models of Associations of Metabolic and CVRFs With Augmentation Index in Youth and Young Adults with T1DM*
Table S3. Generalized Linear Multivariable Models of Associations of Metabolic and CVRFs With Carotid‐Femoral Pulse Wave Velocity in Youth and Young Adults With T1DM*
Table S4. Generalized Linear Multivariable Models of Associations of Metabolic and CVRFs With Femoral‐Foot Pulse Wave Velocity in Youth and Young Adults with T1DM*
Table S5. Generalized Linear Multivariable Models of Associations of Metabolic and CVRFs With Carotid‐Radial Pulse Wave Velocity in Youth and Young Adults with T1DM*
Figure S1. Arterial Stiffness means with standard error bars by glycosylated hemoglobin (HbA1c levels, %) in youth and young adults with type 1 diabetes mellitus adjusted (for mean arterial pressure, age, and sex).


