Figure 2.
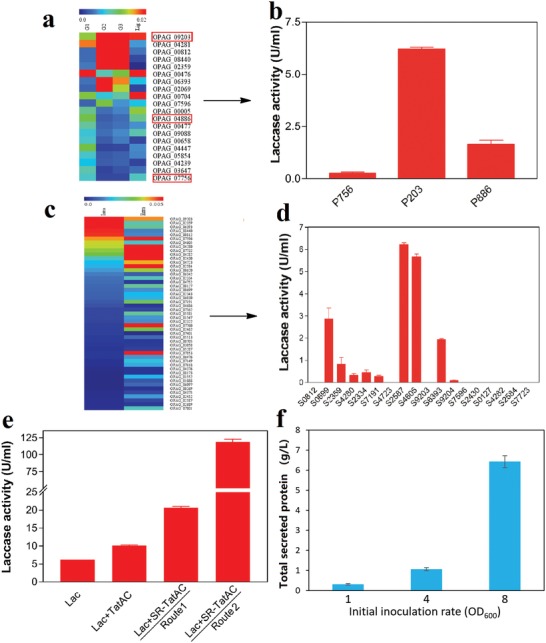
Biodesign of a highly efficient secretory system for heterologous protein production in R. opacus PD630. a) The heatmap showed the expression abundance of 20 highly expressed proteins under different conditions. The proteins were sorted (with the highest expressed on the top) by their average abundance in glucose medium from three different bacterial growth phases: log phase (G1), early stationary phase (G2), and late stationary phase (G3). “Lig” represented the proteins from the bacteria grown in kraft lignin as a carbon source. The proteins highlighted by the red square indicated that their promoter and RBS were predicted and used for laccase heterologous expression as shown in (b). b) The extracellular laccase activity for engineered strain with different promoter/RBS derived from the protein highlighted in (a). c) The heatmap showed the comparison of protein abundance between intracellular (Intra) and extracellular (Extra) proteins for the top 50 highly expressed ones with predicted Tat system signal peptides. d) The extracellular laccase activities for engineered strains with laccase integrated with different signal peptides derived from selected proteins in (c). The value showed in the heatmap represented the relative fold change of each protein among different conditions. e) The extracellular laccase activities for an engineered strain with secretory production of laccase and the engineered secretory machinery under different fermentation conditions to optimize protein folding. “Route 1” represented the conventional fermentation strategy with a normal minimum medium, where the expressed laccase would recruit copper and fold into functional protein in the bacterial cell. “Route 2” represented the optimized fermentation strategy, where the laccase was first expressed on a copper‐deficient medium as apoprotein, and then incubated with copper‐containing medium to refold and restore enzyme activity. All aforementioned laccase activities in this figure were measured from the supernatant of fermentation for engineered strains grown on 1% glucose medium after 4 d with 1.4 g L−1 NH4NO3 as a nitrogen source. f) The yield of secreted protein by the engineered strain PD630_La after 7 d of growth on 6% glucose as carbon source and 2.4 g L−1 NH4NO3 as nitrogen source with different initial strain inoculation rates. Three different inoculation rates were used OD600 1.0, 4.0, and 8.0. The protein yield was calculated according to the weight of isolated proteins from the supernatant.
