Abstract
Micro-CT imaging is a well-established morphological method for the visualization of animal models. We used ethanol fixation of the mouse brains to perform high-resolution micro-CT scans showing in great details brain grey and white matters. It was possible to identify more than 50 neuroanatomical structures on the 5 selected coronal sections. Among white matter structures, we identified fornix, medial lemniscus, crossed tectospinal pathway, mammillothalamic tract, and the sensory root of the trigeminal ganglion. Among grey matter structures, we identified basal nuclei, habenular complex, thalamic nuclei, amygdala, subparts of hippocampal formation, superior colliculi, Edinger–Westphal nucleus, and others. We suggest that micro-CT of the mouse brain could be used for neurohistological lesions evaluation as an alternative to classical neurohistology because it does not destroy brain tissue.
1. Introduction
Microcomputed tomography (micro-CT) scanning provides nondestructive imaging of tissues and has potential to produce 3D images. Highly mineralized structures, such as bones and teeth, give very good contrast in micro-CT [1]. On the other hand imaging of soft tissues such as nerve, muscle, adipose tissue, or ligaments is very problematic [2]. Interestingly, alcohol fixation works well with the neuronal tissue and specifically with the brain but together with the iodine and phosphotungstic acid [3]. Brain tissue has several distinguishing characteristics compared to other soft tissues. It is composed of white matter that contains relatively high amount of phospholipid molecules forming myelin sheaths around axons of neurons that behave on the micro-CT simply as fat (mostly visualized on micro-CT darker compared to grey matter) and from grey matter containing bodies of neurons (appear on micro-CT lighter compared to white matter bundles) that share basic common cellular characteristics (biophysical, biochemical, and biological) of other soft tissues. Micro-CT studies visualizing neuronal tissue usually focus on peripheral nerves and their lesions [4], overall brain atrophy [5], freeze-dried human acellular nerve allografting (hANA) [6], and brain tumor models in mice [7]. Generally there are more micro-CT studies on pathological neuronal tissues than on the healthy ones.
Behavioral studies on the mice model often require precise analysis of the brain area selected for the experiment. For example, in animal models of ischemia exact place of the neuronal lesion has to be verified and quantified [8]. Evaluation of exact lesion site needs different kinds of structural/histological atlases ([9, 10]; Allen Mouse Brain Atlas, 2014) based on various staining procedures (Nissl, parvalbumin, calbindin, etc.). Besides classical neurohistological lesion verification, combination of 7T Bruker MRI with magnetic particle analysis (MPI) of the brain tissue in real time becomes popular [11]. Micro-CT imaging of the brain could be considered as a new attempt to visualize neuronal tissue for the experimental purposes. Micro-CT imaging with phase contrast of the ethanol fixated rat brain was successfully described in [12], although only gross neuroanatomical structures were observed. Soaking the brains in nonionic iodinated contrast agent resulted in clear differences in signal between the grey matter, the white matter, and the ventricular spaces [13], but without possibility to distinguish neuroanatomical borders of individual brain nuclei or cortical regions. Diffusible iodine-based contrast-enhanced computed tomography (diceCT) in female mouse was suggested to be effective for gross differences in the overall brain shape in large numbers of samples [14]. Combined MRI-CT atlases of developing and adult mouse brains fixed with paraformaldehyde and subsequent PBS wash-out are unique for coregistration of brain areas but without detailed neuroanatomical structures delineation [15]. We tried to visualize and identify on micro-CT as much as possible neuroanatomical structures on coronal, sagittal, and horizontal sections of the healthy mouse brain.
2. Materials and Methods
2.1. Tissue Sample Origins
We evaluated 5 brains from C57BL/6 genetically modified male mice (weight 17-20 g) from the Institute of Experimental Imaging, First Faculty of Medicine, Charles University, Prague, Czech Republic. This mouse strain was selected because it is commonly used in neurosciences and other research fields [16]. Mice were euthanized by cervical dislocation and their brains were harvested for purpose of this study; this method did not affect mice brain distortion at all. Study was approved by Ethical Committee of the Third Faculty of Medicine, Charles University, Czech Republic.
2.2. Tissue Sample Processing
Brains from 5 mice were carefully extracted from the skulls by the following steps. Cervical spinal cord and brain stem were released by small tongs as disruption of cervical vertebras. Then temporal bones and vestibulocochlear, oculomotor, optic, and olfactory nerves were dissected. After extraction of the brain from skull, any remnants of bone fragments on the brain surface were carefully checked and cleaned before scanning. The brain samples were put into Eppendorf tubes with ethanol-soaked gauze at the bottom for the purpose of the scan. The conical shape of Eppendorf tubes very efficiently supports the samples and avoids undesirable movements. The wet gauze maintains a saturated gaseous atmosphere preventing further drying out and shrinkage of sample. After the extraction, brains were fixated subsequently in 25%, 50%, 75%, and 97% ethanol for 12 hours. This type of ethanol fixation is also known as graded dehydration series of ethanol (GEHC) and has been documented as promising in undistorted soft tissue fixation [17]. Micro-CT scanning was performed after 168 hours of fixation.
2.3. Tissue Sample Scanning
Brains were left prior to scanning on the gauze for 40 minutes in air temperature 23°C. This allowed vaporization of redundant ethanol from the whole brain, including the ventricles and other cavities. After the period of drying, brains were positioned in the special plastic holder with an ethanol reservoir, which made an atmosphere of gas, which prevented structural changes of the brains during scanning [18, 19]. Two different scanning techniques were performed. First, just X-ray radiography was performed followed by a microtomography and final 3D reconstruction [18, 19]. The data were reconstructed into the final 3D dataset using Volex reconstruction software and visualized using program CTVox in standard PC [20]. On the sagittal projection some processing artifacts are often seen: flattening of the whole brain craniocaudally, artificial space between the hippocampal formation and thalamus, fimbria fornicis separated from the white matter nearby stria terminalis and ventriculus lateralis, and cerebellar fissure behind inferior colliculi.
2.4. Micro-CT Apparatus
The used micro-CT set-up was described in detail in our previous publications [18, 19, 21]. Briefly, apparatus consisted of two different custom-built micro-CT systems; routine detection system was equipped with a Kevex™ PXS-11 X-ray tube and Timepix detector in Quad configuration (four read-out chips with a common silicon sensor providing sensitive area 28 × 28 mm, 512 × 512 pixels, 55 μm pixel pitch). The highest achievable spatial resolution was approximately 28 μm. Presented 2D microradiographic images were acquired with the introduced setup. The other high-resolution system was equipped with a large area photon counting detector WidePIX10×5. WidePIX is a recently introduced technology for tiling of large area PCD arrays from individual Timepix chips [22]. Specifically, detector WidePIX10x5 is composed of 50 Timepix tiles and offers approximately 140 x 70 mm field view (2560 × 1280 pixels). High quality microfocus X-ray tube Hamamatsu L8601-01 enables spatial resolution down to 5 micrometers [23].
2.5. Scan Parameters
The high-resolution setup was used for the presented micro-CT scans. The data were acquired with an emphasis on high CNR. The acquisition time was adjusted in order to reach at least 105 or 104 detected photons per pixel in the background of the object in microradiography or a micro-CT projection, respectively. CT reconstructions were done by Volex reconstruction engine (courtesy of Fraunhofer IIS and Technology, Germany).
The detector as well as the whole CT scan was controlled using Pixelman software [24]. The CT scan was carried out with 4.4 μm EPS. The total number of 848 projections was acquired with 0.38 degree angle step. The acquisition time was 3.6 seconds per projection. The tube voltage was set to 60 kVp and it was operated with 6 W of output power. The projections were processed using a dedicated beam-hardening correction [25] and the slight image distortions coming from the tiled detector construction were corrected [26]. The CT reconstruction was carried out using Volex reconstruction engine.
2.6. Gray and White Matter Labelling
For the frontal and sagittal sections Allen Mouse Atlas was used as reference [27]. For the horizontal sections C57BL/6J Atlas was used as reference (The Mouse Brain Library, http://www.mbl.org). Anatomical structures of digitalized brain sections were labeled in the environment of freeware program Xnview (https://www.xnview.com/en/). All depicted pictures of the labelled mouse brain are from one specimen only so that structures correspond between exactly three planes (horizontal, sagittal, and coronal), because of tiny morphological differences between various mouse brains.
3. Results
We identified 42 white matter and 53 grey matter brain structures (see Abbreviations) in five coronal (Figure 1), four sagittal (Figure 2), and three horizontal (Figure 3) brain sections of ex vivo healthy mouse brain using micro-CT. All structures were identified manually by two experienced neuroanatomists and registered in micro-CT scans using online Adult Mouse Brain Atlas [27] for coronal and sagittal sections and the online Mouse Brain Library (C57BL/6J Atlas) for horizontal sections.
Figure 1.
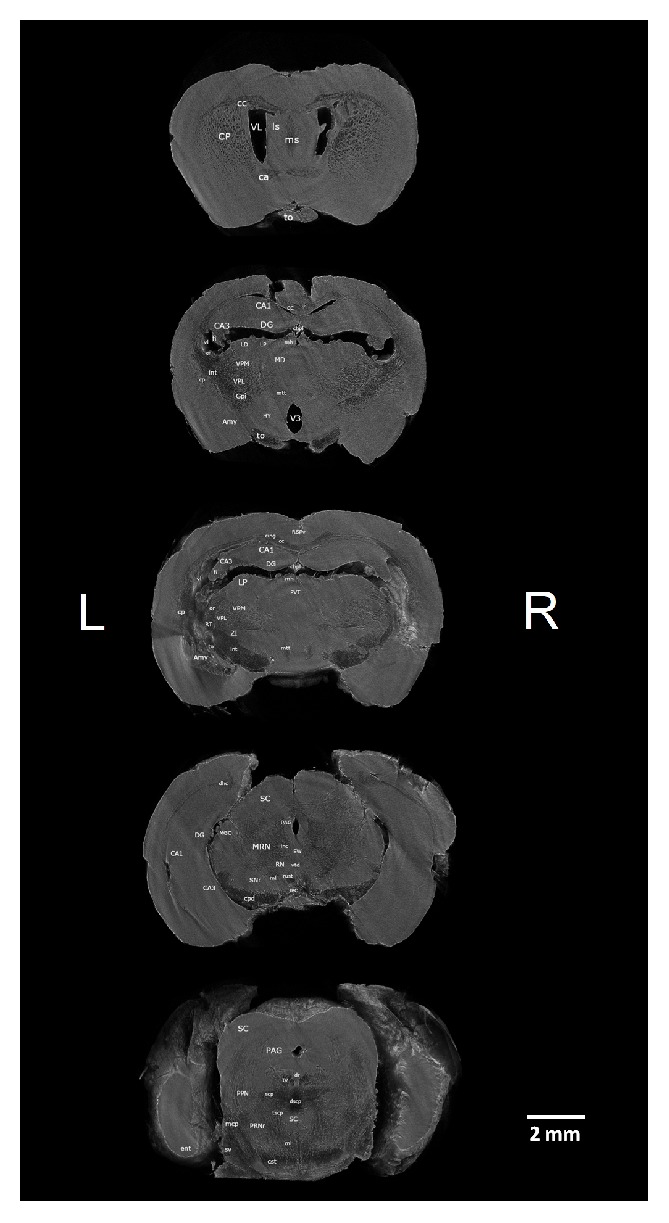
Micro-CT slices (program CTVox) of the 5 coronal sections of the mouse brain with labeled grey and white matter structures. Brain sections from top down are taken 1x at the level of anterior commissure, 2x at the dorsal hippocampus, 1x at the ventral hippocampus, and 1x at the brain stem (superior colliculi). Section orientation: L – left, R – right.
Figure 2.
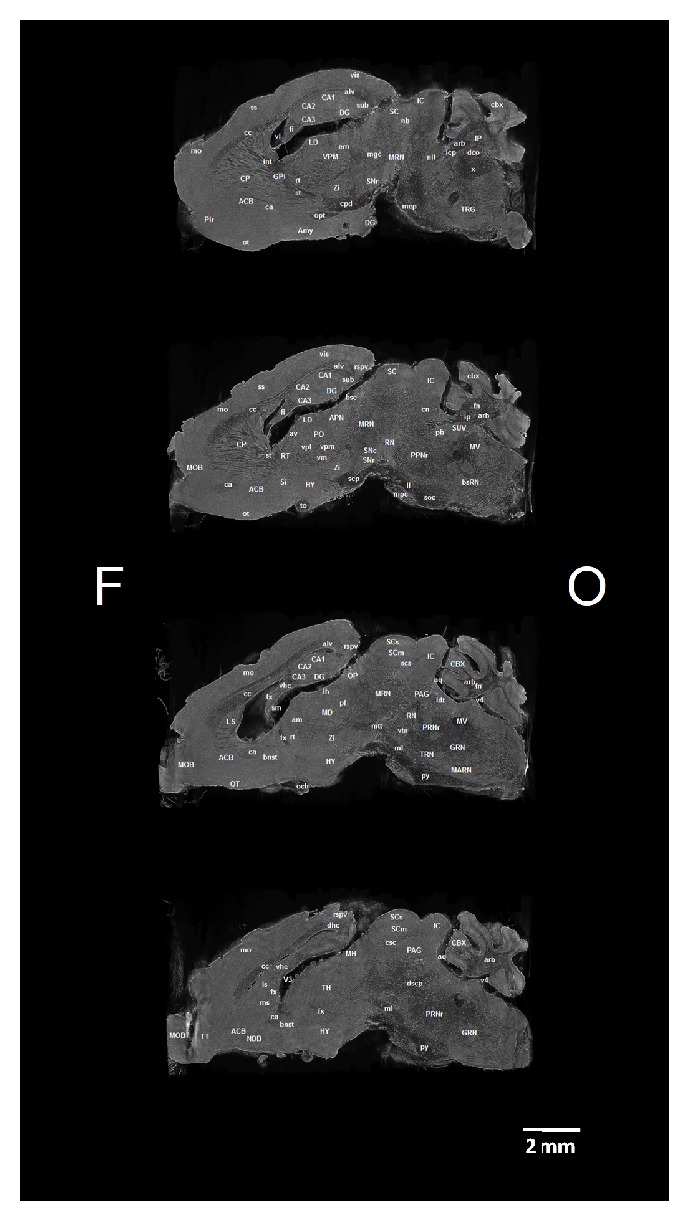
Micro-CT slices (program CTVox) of the 4 sagittal sections of the mouse brain with labeled grey and white matter structures. Brain sections from top down are taken 1x at the level of pallidum internum, 1x at the middle of caudatoputamen, 1x at the lateral septum, and 1x at the medial septum. Section orientation: F – frontal, O – occipital.
Figure 3.
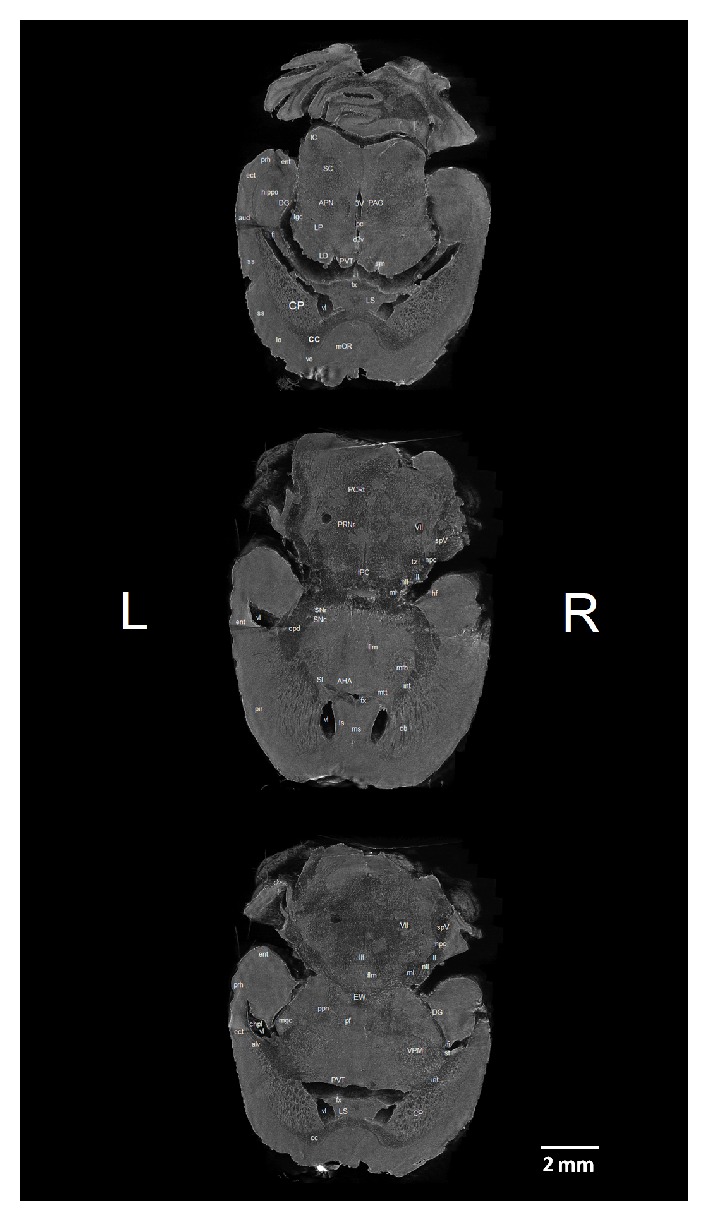
Micro-CT slices (program CTVox) of the 3 horizontal sections of the mouse brain with labeled grey and white matter structures. Brain sections from top down are taken 1x at the upper part of thalamic paraventricular nucleus, 1x at the anterior hypothalamic area, and 1x at the lower part of the thalamic paraventricular nucleus. Section orientation: L – left, R – right.
3.1. Frontal Sections of the Mouse Brain
The positions of the five coronal sections of the mouse brain were selected because of their relevance to experimental neuropsychological studies in animal model. In frontodorsal order the sections were taken (a) in the frontal lobe at the level of the anterior commissure, (b) at the ventral part of the dorsal dentate gyrus, dorsal hippocampus, and the third ventricle, (c) at the dorsal part of the dorsal dentate gyrus, dorsal hippocampus, and the paraventricular nucleus of the thalamus, (d) at the level of the ventral dentate gyrus, ventral hippocampus, and midbrain reticular nucleus, and (e) at the level of the brain stem with superior colliculi and dorsal raphe nucleus. We were unable to identify hyperintensity in the brain stem between trigeminal nuclear complex, lateral lemniscal nuclei, and medial cerebellar peduncle. Another poorly visible area is located below superior and inferior colliculi, towards thalamic nuclei. Similarly, resolution of the bed nucleus striae terminalis and substantia innominata is poor. Opposite, there is good resolution for both zona incerta and reticular part of the substantia nigra. Although there is relatively big trigeminal nuclear complex that is easy to identify, separate subnuclei of the complex are hard to differentiate. The caudatoputamen is a very well visible structure and inside is rich system of the hypointensities that could be either white matter of the internal capsule or the Virchow-Robin spaces formed by capillary bed stream of thalamostriatal artery. Hypothalamic subparts are more difficult to discern compared to thalamic subnuclear groups. Stria medullaris thalami is normally found on the superior part of the thalamus; however here it is detached from it and bound to dorsal hippocampal commissure on the caudal surface of the fornix. While most of the thalamic nuclei are hyperdense, most midbrain structures (reticular nucleus, periaqueductal gray, etc.) are hypodense.
3.2. Sagittal Sections of the Mouse Brain
Within hippocampal formation dentate gyrus, CA1, Ca2, and CA3 subfields and subiculum were identified. Sagittal projection offers better visibility over frontal projection. Above cerebellar peduncle zona incerta, substantia nigra, and ventrally stria terminalis were visible. Within brain stem trigeminal nucleus, dorsal vagal nucleus, and nucleus interpeduncularis were visible. On the other hand, we have not seen well borders of the amygdaloid complex; it had the same gray color as nearby structures (olfactory tubercle or nucleus accumbens).
3.3. Horizontal Sections of the Mouse Brain
We identified in the ventral part of the sections clearly visible medial and lateral septum. Callosal body in contrast to online atlas was very well visible in all three sections and in front of it was well preserved medial frontal and/or orbital cortex. Also frontal part of the lateral ventricles clearly separated caudatoputamen from septal nuclei and internal capsule. Penetrations of the internal capsule into caudatoputamen are, especially, well visible. Dorsally to septal area structures of the thalamus (paraventricular nucleus and laterodorsal complex of nuclei) were located, and also stria medullaris thalami and lateral geniculate complex. On the other hand, detailed inner structure of the hippocampal formation (ventral part) was not very well visible as in the frontal sections. Besides reticular, pontine, or parvocellular nuclei, other brain stem nuclei were not visible compared to the frontal sections.
4. Discussion
Micro-CT imaging in mouse is often limited to the whole body scans, including skeleton, organs and blood vessels [28, 29], and brain blood supply changes in various experimental pathological conditions [30, 31], or to the brain tumors (for example glioblastoma) [32]. Scanning of the mouse brain gives better results when it is extracted from the skull. The reason is that the skull induces beam-hardening artifacts to adjacent soft tissue [15]. Our work provides comparable results as reported by [3]; nevertheless, in our case, any high-Z contrast agent was needed.
High-resolution MRI three-dimensional atlas of the mouse brain shows sixty-two structures at the resolution 32 μm with the habenular nuclear complex being the smallest visible structure [33]. In comparison our micro-CT ethanol fixated brain scans showed more than fifty structures within only 5 representative coronal sections. It seems that pure GEHC ethanol brain fixation shows better differences between white and gray matters on micro-CT compared to MRI. We identified small white matter structures like cingulum bundle, medial lemniscus, crossed tectospinal pathway, and stria terminalis which is a better result compared to the MRI.
Frontal and sagittal sections atlases of the mouse brain [27] are easier to find in the literature compared to the horizontal ones (for example MRI atlas of C57BL/6J, DBA/2J, or A/J mouse from The Mouse Brain Library). Some MRI atlases are without grey and white brain matter labels, for example, 8-week-old 129S1/SvImJ male mice atlas [34].
We did not attempt yet to create the whole atlas of the mouse brain on the micro-CT. Our goal was to visualize clinically important brain structures like hippocampal formation and its subfields, thalamic nuclei, fornix, medial and lateral septal nuclei, and others. We suggest as the next step manual or semiautomatic reconstruction of the whole mouse brain micro-CT atlas. Histological staining (Nissl) and optical microscopy are mostly used for brain lesion evaluation in experimental studies (insertions of cannulas, electrolesions, chemical lesions, electrode positions, polymer substance delivery, ischemia after carotid arteries ligations, etc.). The disadvantage of these approaches is altered brain tissue that cannot be used afterwards for other staining (for example immunostaining) or at the cost of complicated protocols for sections storing and handling. With the micro-CT lesion verification, we can use intact brain tissue for further processing and thus replacing classical histological verification with virtual visual evaluation. Moreover, micro-CT lesion visualization can be enhanced by computer processing leading to volume rendering or providing virtual dissection of the brain in unorthodox planes unavailable in classical histology. Level of details in high-resolution micro-CT almost corresponds to the classical histology sections. Within destructive methodologies, it seems to be a choice for immunohistochemistry since the brains are processed only in ethanol.
5. Conclusion
We show that micro-CT could be used in neuroresearch alongside classical histology or magnetic resonance imaging. Besides higher price and lower resolution of the magnetic resonance imaging, it is not always available to all laboratories and micro-CT is easier to get access to. Even if one does not have micro-CT in the laboratory, it is possible to use fixation of the brain specimen and send it to micro-CT for analysis. This is not so simple for magnetic resonance imaging; we cannot use fixation or it would not be visualized properly. Fixation of the brain tissue should be done as soon as possible or the brain would decompose. Magnetic resonance imaging is better for living organisms while micro-CT for fixed brain tissue. Laboratories with micro-CT could offer services for others (sending fixated brain specimen) since acquisition time for micro-CT scanning is relatively short compared to magnetic resonance imaging. Immunohistochemistry or general staining histological protocols could then follow in a short time. The disadvantage of the micro-CT is still the relatively small Timepix detector area but with time we could expect an increase in its size. Ex vivo ethanol fixation of the brain tissue grants sufficient tissue contrast, but we trend to the situation where brain rotation could be highly contrasted even during in vivo scanning.
Acknowledgments
Project was supported by Charles University [Grants nos. Q35, Q41, and 260388/SVV/2019]; European Regional Development Fund-Project “Engineering applications of micro world physics” [Grants nos. CZ.02.1.01/0.0/0.0/16_019/0000766]; the Charles University Grant Agency [Grant no. GAUK 130717]; Czech Science Foundation [Grant no. P30412G069]; National Institute of Mental Health (NIMH-CZ) [Grant no. ED2.1.00/03.0078]; and the European Regional Development Fund [Grant no. RVO67985823].
Abbreviations
- ACB:
Nucleus accumbens
- AHA:
Anterior hypothalamic area
- alv:
Alveus
- Amy:
Amygdala
- APN:
Anterior pretectal nucleus
- aq:
Cerebral aqueduct
- arb:
Arbor vitae
- aud:
Auditory cortex
- av:
Anteroventral nucleus of the thalamus
- bnst:
Bed nucleus striae terminalis
- bsc:
Brachium of superior colliculus
- bsRN:
Brain stem reticular nuclei
- ca:
Anterior commissure
- CA1:
Field of CA1
- CA3:
Field of CA3
- cb:
Cell bridges of ventral striatum
- cbx:
Cerebellar cortex
- cc:
Corpus callosum
- cing:
Cingulum bundle
- cn:
Cuneiform nucleus
- CP:
Caudatoputamen
- cpd:
Cerebral peduncle
- cst:
Corticospinal tract
- d3v:
Dorsal third ventricle
- dco:
Dorsal cochlear nucleus
- DG:
Dentate gyrus
- dhc:
Dorsal hippocampal commissure
- dr:
Dorsal nucleus raphe
- dscp:
Superior cerebellar peduncle decussation
- ect:
Ectorhinal cortex
- em:
External medullary lamina of the thalamus
- ent:
Entorhinal area
- EW:
Edinger-Westphal nucleus
- fi:
Fimbria fornicis
- flm:
Medial longitudinal fascicle
- fn:
Fastigial nucleus
- fx:
Fornix
- Gpi:
Globus pallidus, internal segment
- GRN:
Gigantocellular reticular nucleus
- hf:
Hippocampal fimbria
- hippo:
Hippocampal formation
- HY:
Hypothalamus
- chpl:
Choroid plexus
- IC:
Inferior colliculus
- icp:
Inferior cerebellar peduncle
- III:
Oculomotor nucleus
- inc:
Interstitial nucleus of Cajal
- int:
Internal capsule
- IP:
Interposed nucleus of cerebellum
- IPC:
Interpeduncular nucleus
- iv:
Trochlear nucleus
- LAV:
Lateral vestibular nucleus
- LD:
Lateral dorsal nucleus of the thalamus
- ldt:
Laterodorsal tegmental nucleus
- lgc:
Lateral geniculate complex
- lh:
Lateral habenula
- LHA:
Lateral hypothalamic area
- ll:
Lateral lemniscus
- lo:
Lateral orbital cortex
- LP:
Lateral posterior nucleus of the thalamus
- LPO:
Lateral preoptic area
- ls:
Lateral septal nucleus
- MARN:
Magnocellular reticular nucleus
- mcp:
Middle cerebellar peduncle
- MD:
Mediodorsal nucleus of the thalamus
- mfb:
Medial forebrain bundle
- MGC/mgc:
Medial geniculate complex
- mh:
Medial habenula
- ml:
Medial lemniscus
- mo:
Somatomotor cortical areas
- MOB:
Main olfactory bulb
- mOR:
Medial orbital cortex
- mpc:
Medial cerebellar peduncle
- MRN:
Midbrain reticular nucleus
- ms:
Medial septal nucleus
- mtt:
Mammillothalamic tract
- MV:
Medial vestibular nucleus
- MV:
Medial vestibular nucleus
- nb:
Nucleus of the brachium of the inferior colliculus
- NDB:
Nucleus of the diagonal band
- nll:
Nucleus of the lateral lemniscus
- not:
Nucleus of the optic tract
- och:
Optic chiasma
- OP:
Olivary pretectal nucleus
- or:
Optic radiation
- ot:
Olfactory tubercle
- PAG:
Periaqueductal gray
- pb:
Parabrachial nucleus
- pc:
Posterior commissure
- PCRt:
Parvicellular reticular nucleus
- pf:
Parafascicular nucleus
- Pir:
Piriform cortical area
- Po:
Posterior thalamic complex
- PPN/ppn:
Pedunculopontine nucleus
- ppt:
Posterior pretectal nucleus
- prh:
Perirhinal cortex
- PRNr:
Pontine reticular nucleus
- PVT:
Paraventricular nucleus of the thalamus
- py:
Pyramid
- RN:
Red nucleus
- RSPv/rspv:
Retrosplenial area, ventral part
- RT/rt:
Reticular nucleus of the thalamus
- rust:
Rubrospinal tract
- SC:
Superior colliculus
- SCm:
Motor part of superior colliculus
- scp:
Superior cerebellar peduncles
- SCs:
Sensory part of superior colliculus
- scs:
Superior colliculus commissure
- SI:
Substantia innominata
- sm:
Stria medullaris thalami
- SNc:
Substantia nigra, compact part
- SNr:
Substantia nigra, reticular part
- soc:
Superior olivary complex
- spV:
Spinal tegmental tract
- ss:
Somatosensory cortical areas
- st:
Stria terminalis
- stn:
Subthalamic nucleus
- sub:
Subiculum
- SUV:
Superior vestibular nucleus
- SV:
Sensory root of the trigeminal nerve
- TH:
Thalamus
- to:
Tractus opticus
- TRG:
Trigeminal nuclei
- TRN:
Tegmental reticular nucleus
- tscp:
Crossed tectospinal pathway
- TT:
Taenia tecta
- tz:
Trapezoid body
- V3:
Third ventricle
- v4:
Fourth ventricle
- vhc:
Ventral hippocampal commissure
- VII:
Motor nucleus of the facial nerve
- vIIIn:
Vestibulocochlear nerve
- vis:
Visual cortical areas
- VL/vl:
Lateral ventricle
- vm:
Ventral medial nucleus of the thalamus
- vo:
Ventral orbital cortex
- VPL:
Ventral posterolateral nucleus of the thalamus
- VPM/vpm:
Ventral posteromedial nucleus of the thalamus
- vta:
Ventral tegmental area
- vtd:
Ventral tegmental decussation
- x:
Nucleus X
- Zi/zi:
Zona incerta.
Data Availability
Pictures of the mouse brain are available on request in our laboratory. The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Jud C., Schaff F., Zanette I., Wolf J., Fehringer A., Pfeiffer F. Dentinal tubules revealed with X-ray tensor tomography. Dental Materials. 2016;32(9):1189–1195. doi: 10.1016/j.dental.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Naveh G. R., Brumfeld V., Dean M., Shahar R., Weiner S. Direct MicroCT imaging of non-mineralized connective tissues at high resolution. Connective Tissue Research. 2014;55(1):52–60. doi: 10.3109/03008207.2013.867333. [DOI] [PubMed] [Google Scholar]
- 3.Zikmund T., Novotná M., Kavková M., et al. High-contrast differentiation resolution 3D imaging of rodent brain by X-ray computed microtomography. Journal of Instrumentation. 2018;13, article C02039:1–12. [Google Scholar]
- 4.Hopkins T. M., Heilman A. M., Liggett J. A., et al. Combining micro-computed tomography with histology to analyze biomedical implants for periphera nerve repair. Journal of Neuroscience Methods. 2015;30(255):122–130. doi: 10.1016/j.jneumeth.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buytaert J., Goyens J., De Greef D., Aerts P., Dirckx J. Volume shrinkage of bone, brain and muscle tissue in sample preparation for micro-ct and light sheet fluorescence microscopy (LSFM) Microscopy and Microanalysis. 2014;20(4):1208–1217. doi: 10.1017/S1431927614001329. [DOI] [PubMed] [Google Scholar]
- 6.Zhu S., Zhu Q., Liu X., et al. Three-dimensional reconstruction of the microstructure of human acellular nerve allograft. Scientific Reports. 2016;1(6, article 30694) doi: 10.1038/srep30694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yahyanejad S., Granton P. V., Lieuwes N. G., et al. Complementary use of bioluminescence imaging and contrast-enhanced micro-computed tomography in an orthotopic brain tumor model. Molecular Imaging. 2014;13 doi: 10.2310/7290.2014.00038. [DOI] [PubMed] [Google Scholar]
- 8.Fluri F., Schuhmann M. K., Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Design, Development and Therapy. 2015;2(9):3445–3454. doi: 10.2147/DDDT.S56071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates. 4th. Cambridge, Mass, USA: Academic Press; 2012. [Google Scholar]
- 10.Jacobowitz D. M., Abbot L. C. Chemoarchitectonic Atlas of the Developing Mouse Brain. 1st. Boca Raton, Fla, USA: CRC Press; 1997. [Google Scholar]
- 11.Zheng B., Vazin T., Goodwill P. W., et al. Magnetic particle imaging tracks the long-term fate of in vivo neural cell implants with high image contrast. Scientific Reports. 2015;5, article 14055 doi: 10.1038/srep14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda T., Thet-Lwin T., Kunii T., et al. Ethanol fixed brain imaging by phase-contrast X-ray technice. Journal of Physics: Conference Series. 2013;425(2, article 022004) [Google Scholar]
- 13.Saito S., Murase K. Ex vivo imaging of mouse brain using micro-CT with non-ionic iodinated contrast agent: a comparison with myelin staining. British Journal of Radiology. 2012;85(1019):e973–e978. doi: 10.1259/bjr/13040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson R., Maga A. M. A novel procedure for rapid imaging of adult mouse brains with microCT using iodine-based contrast. PLoS ONE. 2015;10(11, article e0142974) doi: 10.1371/journal.pone.0142974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aggarwal M., Zhang J., Miller M., Sidman R., Mori S. Magnetic resonance imaging and micro-computed tomography combined atlas of developing and adult mouse brains for stereotaxic surgery. Neuroscience. 2009;162(4):1339–1350. doi: 10.1016/j.neuroscience.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H. W. The Allen Reference Atlas: A Digital Color Brain Atlas of the C57Bl/6J Male Mouse. New Jersey, NJ, USA: John Wiley and Sons Inc; 2008. [Google Scholar]
- 17.Metscher B. D. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiology. 2009;9(11) doi: 10.1186/1472-6793-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudak J., Zemlicka J., Karch J., et al. High-contrast X-ray micro-radiography and micro-CT of ex-vivo soft tissue murine organs utilizing ethanol fixation and large area photon-counting detector. Scientific Reports. 2016;27(6, article 30385) doi: 10.1038/srep30385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudak J., Zemlicka J., Krejci F., et al. Evaluation of sample holders designed for long-lasting X-ray micro-tomographic scans of ex-vivo soft tissue samples. Journal of Instrumentation. 2016;11, article C03005 [Google Scholar]
- 20.Bruker MicroCT. Volume rendering. 2016, http://bruker-microct.com/products/ctvox.htm.
- 21.Dudak J., Zemlicka J., Krejci F., et al. X-ray micro-CT scanner for small animal imaging based on Timepix detector technology. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2015;773:81–86. [Google Scholar]
- 22.Jakubek J., Jakubek M., Platkevic M., et al. Large area pixel detector WIDEPIX with full area sensitivity composed of 100 Timepix assemblies with edgeless sensors. Journal of Instrumentation. 2014;9(4, article C04018) [Google Scholar]
- 23.Hamatsu datasheet. https://www.hamamatsu.com/resources/pdf/etd/L9181-02_TXPR1015E.pdf.
- 24.Turecek D., Holy T., Jakubek J., Pospisil S., Vykydal Z. Pixelman: a multi-platform data acquisition and processing software package for Medipix2, Timepix and Medipix3 detectors. Journal of Instrumentation. 2011;6(1) C01046-C01046. [Google Scholar]
- 25.Jakubek J. Data processing and image reconstruction methods for pixel detectors. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2007;576(1):223–234. doi: 10.1016/j.nima.2007.01.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zemlicka J., Dudak J., Karch J., Krejci F. Geometric correction methods for Timepix based large area detectors. Journal of Instrumentation. 2017;12(1) C01021-C01021. [Google Scholar]
- 27.Allen Brain Reference Atlases. Adult mouse. 2004, http://atlas.brain-map.org/
- 28.Wang H., Stout D. B., Taschereau R., et al. MARS: A mouse atlas registration system based on a planar x-ray projector and an optical camera. Physics in Medicine and Biology. 2012;57(19):6063–6077. doi: 10.1088/0031-9155/57/19/6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baiker M., Milles J., Dijkstra J., et al. Atlas-based whole-body segmentation of mice from low-contrast Micro-CT data. Medical Image Analysis. 2010;14(6):723–737. doi: 10.1016/j.media.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Ghanavati S., Yu L. X., Lerch J. P., Sled J. G. A perfusion procedure for imaging of the mouse cerebral vasculature by X-ray micro-CT. Journal of Neuroscience Methods. 2014;221:70–77. doi: 10.1016/j.jneumeth.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Park J. Y., Lee S. K., Kim J. Y., et al. A new micro-computed tomography-based high-resolution blood-brain barrier imaging technique to study ischemic stroke. Stroke. 2014;45(8):2480–2484. doi: 10.1161/STROKEAHA.114.006297. [DOI] [PubMed] [Google Scholar]
- 32.Kirschner S., Mürle B., Felix M., et al. Imaging of orthotopic glioblastoma xenografts in mice using a clinical CT scanner: comparison with micro-CT and histology. PLoS ONE. 2016;9(11, article e0165994):p. 11. doi: 10.1371/journal.pone.0165994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorr A., Lerch J., Spring S., Kabani N., Henkelman R. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. NeuroImage. 2008;42(1):60–69. doi: 10.1016/j.neuroimage.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 34.Kovacević N., Henderson J. T., Chan E., et al. A three-dimensional MRI atlas of the mouse brain with estimates of the average and variability. Cerebral Cortex. 2005;15(5):639–45. doi: 10.1093/cercor/bhh165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Pictures of the mouse brain are available on request in our laboratory. The data used to support the findings of this study are available from the corresponding author upon request.


