Figure 1.
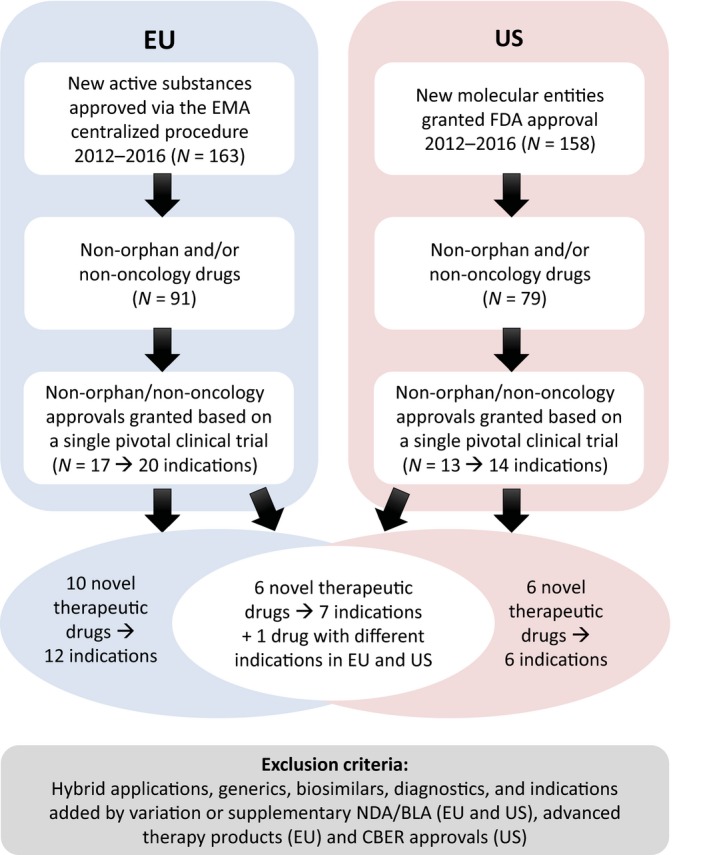
Selection criteria for the analysis. Overview of the selection criteria for identification of novel therapeutic drugs for which one or more indications were approved based on a single pivotal clinical trial in the United States (US) and European Union (EU) between 2012 and 2016. CBER, Center for Biologics Evaluation and Research; FDA, US Food and Drug Administration; EMA, European Medicines Agency; NDA/BLA, new drug application/biologics license application.
