Figure 2.
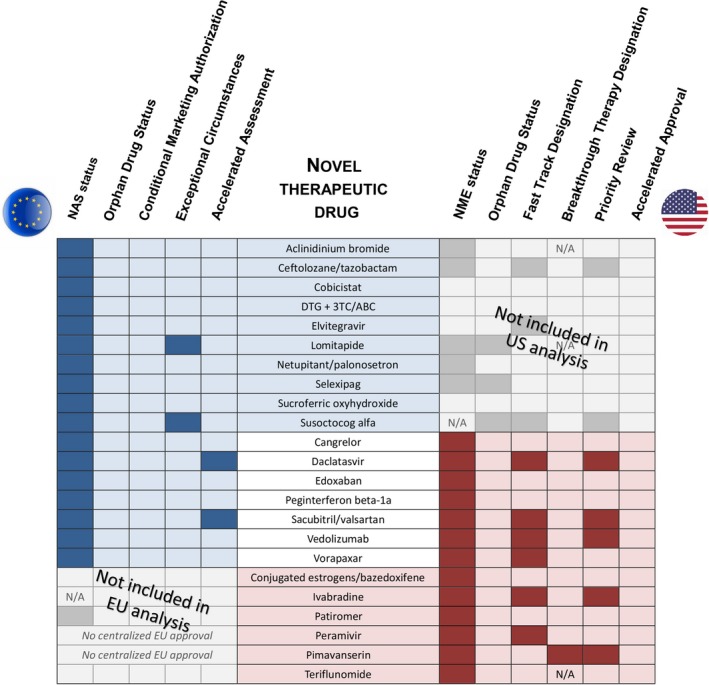
Regulatory overview of novel therapeutic drugs approved based on a single pivotal trial. Overview of regulatory pathways and designations for novel therapeutic drugs for which one or more indications were approved based on a single pivotal trial by the US Food and Drug Administration Center for Drug Evaluation and Research and/or via the European Medicines Agency Centralized Procedure between 2012 and 2016. Color shading signifies drugs fulfilling study selection criteria in the European Union (EU) only (blue shading), in both regions (no shading), and United States (US) only (red shading). 3TC, Lamivudine; ABC, Abacavir; DTG, dolutegravir; NAS, New Active Substance; NME, New Molecular Entity; N/A, not applicable.
